TITLE 1. ADMINISTRATION
DEPARTMENT OF VETERANS SERVICES
Final Regulation
REGISTRAR'S NOTICE: The
Department of Veterans Services is claiming an exemption from the
Administrative Process Act in accordance with § 2.2-4002 A 25 of the Code
of Virginia, which excludes the department when promulgating regulations
pursuant to § 58.2-3219.7 of the Code of Virginia.
Title of Regulation: 1VAC80-10. Disabled Veteran Real
Property Tax Exemption (adding 1VAC80-10-10 through 1VAC80-10-100).
Statutory Authority: § 58.1-3219.7 of the Code of
Virginia.
Effective Date: September 18, 2017.
Agency Contact: Carrie Ann Alford, Director of Policy
and Planning, Department of Veterans Services, 101 North 14th Street, 17th
Floor, Richmond, VA 23219, telephone (804) 225-4716, or email carrieann.alford@dvs.virginia.gov.
Summary:
The regulation establishes the requirements of the
application process and implementation of the real property tax exemption for
100% disabled veterans and their surviving spouses.
CHAPTER 10
100% DISABLED VETERAN REAL PROPERTY TAX EXEMPTION
1VAC80-10-10. Definitions.
The following words and terms when used in this chapter
shall have the following meanings unless the context clearly indicates
otherwise:
"Commissioner" means the Commissioner of the
Department of Veterans Services.
"Department" means the Virginia Department of
Veterans Services.
"Dwelling" means the single structure, including
any permanent attachments thereto, that is the principal place of residence of
the qualifying veteran or surviving spouse.
"Exemption" means the exemption from real
property taxes authorized by subdivision (a) of Section 6-A of Article X of the
Constitution of Virginia and § 58.1-3219.5 of the Code of Virginia.
"Qualified veteran" is a veteran who has been
rated by the U.S. Department of Veterans Affairs, or any successor agency, to
have a 100% service-connected, permanent, and total disability. If a 100%
disability rating is not permanent (i.e., has not been finally adjudicated or
is scheduled to be reviewed at a future date), the exemption does not apply.
"Real property" is land and anything growing on
it, attached to it, or erected on it, excluding anything that may be severed
without injury to the land, and the dwelling occupied by the qualified veteran
or surviving spouse.
"Surviving spouse" is a spouse of any member of
the armed forces of the United States as determined by the U.S. Department
of Defense, who has been rated by the U.S. Department of Veterans Affairs,
or any successor agency, to have a 100% service-connected, permanent, and total
disability.
"VA" means the U.S. Department of Veterans
Affairs or any successor agency.
1VAC80-10-20. Real property exempt from taxation.
A. The dwelling that is the principal residence of a
qualified veteran, plus up to one acre of land, or more than one acre if a
given locality has exempted such larger acreage pursuant to § 58.1-3210 of
the Code of Virginia, shall be exempt. The exemption extends to real property
improvements other than a dwelling, including the land upon which such
improvements are situated, so long as the principal use of the improvement is
(i) to house or cover motor vehicles or household goods and other personal
effects as classified in subdivision A 14 of § 58.1-3503 of the Code of
Virginia and as listed in § 58.1-3504 of the Code of Virginia and (ii) for
other than a business purpose.
B. Manufactured homes, as defined in § 46.2-100 of
the Code of Virginia, whether or not the wheels and other equipment previously
used for mobility have been removed, shall be exempt after the qualifying
veteran has titled the home in the Commonwealth and shown proof of ownership.
Sections 58.1-3219.5 and 58.1-3219.9 of the Code of Virginia are the only
instances when manufactured homes may be classified as real property. If the
veteran does not own the land on which the manufactured home is located, then
the land is not exempt. The veteran or spouse must meet all other provisions of
§ 58.1-3219.5.
1VAC80-10-30. Full exemption; joint ownership; trusts.
A. The full exemption is authorized when the dwelling and
land are held by a veteran alone or in conjunction with the veteran's spouse as
tenant or tenants for life or joint lives. The exemption does not apply if the
qualified veteran is not on the deed, except when the real property is held in
one of the trusts listed in subsection B of this section.
B. The full exemption is authorized when the real property
is held in one of the following trusts: (i) revocable inter vivos trust over
which the veteran or the veteran and veteran's spouse hold the power of
revocation or (ii) an irrevocable trust under which a veteran alone or in
conjunction with veteran's spouse possesses a life estate or an estate for
joint lives or enjoys a continuing right of use or support. The exemption does
not apply to any other forms of trust or any interest held under a leasehold or
term of years.
1VAC80-10-40. Partial exemptions.
A. If the veteran's 100% disability rating occurs after
January 1, 2011, and the veteran owns a qualified primary residence on the date
of the rating, then the tax exemption begins on the date of such rating.
B. If the qualified veteran acquires the property after
January 1, 2011, then the exemption shall begin on the date of acquisition, and
the previous owner may be entitled to a refund for a pro rata portion of real
property taxes paid pursuant to § 58.1-3360 of the Code of Virginia.
1VAC80-10-50. Surviving spouse exemption.
A. The surviving spouse of a veteran eligible for the
exemption shall also qualify for the exemption, so long as:
1. The death of the qualified veteran occurs on or after
January 1, 2011;
2. The surviving spouse was married to the qualified
veteran at the time of the veteran's death;
3. The surviving spouse does not remarry;
4. The surviving spouse continues to occupy the real
property as the surviving spouse's principal place of residence; and
5. The veteran was eligible for the exemption at the time
of the veteran's death. This exemption is available even if the qualifying
veteran never requested the exemption.
B. The exemption for a surviving spouse includes real
property (i) held by the veteran's spouse as tenant for life, (ii) held in a
revocable inter vivos trust over which the surviving spouse holds the power of
revocation, or (iii) held in an irrevocable trust under which the surviving
spouse possesses a life estate or enjoys a continuing right of use or support.
The exemption does not apply to any other forms of trust or any interest held
under a leasehold or term of years.
1VAC80-10-60. Proration when not all owners qualify for the
exemption.
In the event that the primary residence is jointly owned
by two or more individuals, not all of whom qualify for the exemption pursuant
to subsection A or B of § 58.1-3219.5 of the Code of Virginia, and no person is
entitled to the exemption under this section by virtue of holding the property
in any of the three ways set forth in subsection D of § 58.1-3219.5 of the
Code of Virginia, then the exemption shall be prorated by multiplying the
amount of the exemption or deferral by a fraction that has as a numerator the
percentage of ownership interest in the dwelling held by all such joint owners
who qualify for the exemption pursuant to subsections A and B of § 58.1-3219.5
of the Code of Virginia, and as a denominator, 100%.
1VAC80-10-70. Cooperative associations.
The exemption does not apply to property owned by a
cooperative association or any other form of ownership in which the qualified
veteran does not actually own the real property other than the trusts detailed
in 1VAC80-10-30.
1VAC80-10-80. Qualified veterans and surviving spouses
residing in hospitals, nursing homes, convalescent homes, or other care
facilities.
If the qualified veteran or surviving spouse is residing
in a hospital, nursing home, convalescent home, or other facility for physical
or mental care for an extended period of time, the exemption will continue on
the property so long as such real estate is not used by or leased to others for
consideration.
1VAC80-10-90. Application.
A. A veteran claiming the real property tax exemption
shall file with the Commissioner of the Revenue, or other assessing official,
in the veteran's respective locality:
1. A summary of benefits letter issued by the VA, or its
successor agency, indicating that the veteran has a 100% service-connected,
permanent, and total disability;
2. An affidavit or application on a form provided by the
locality that:
a. Sets forth the name of the veteran and the name of the
spouse, if any, also occupying the real property;
b. Indicates whether the real property is jointly owned by
two spouses; and
c. Certifies that the real property is occupied as the
veteran's principal place of residence; and
3. Proof of residence occupancy acceptable to the
applicable locality, such as a valid Virginia driver's license or other proof
of residency acceptable to the locality.
B. A surviving spouse of a veteran claiming the real
property tax exemption shall file with the Commissioner of the Revenue, or
other assessing official, in the surviving spouse's respective locality:
1. A summary of benefits letter issued by the VA, or its
successor agency, indicating that the veteran had a 100% service-connected,
permanent, and total disability;
2. An affidavit or application on a form provided by the locality
that:
a. Sets forth the name of the deceased veteran and the name
of the spouse;
b. Indicates whether the real property is jointly owned by
the two spouses; and
c. Certifies that the real property is occupied as the
surviving spouse's principal place of residence;
3. Proof of residence occupancy acceptable to the
applicable locality, such as a valid Virginia driver's license, or other proof
of residency acceptable to the locality;
4. Death certification to confirm veteran's date of death
is on or after January 1, 2011; and
5. A certificate of marriage from the appropriate state
office of records.
C. The veteran or surviving spouse may complete the local
tax exemption application before receipt of the VA Summary of Benefits letter.
The Commissioner of Revenue, or other assessing official, shall ensure that the
veteran is aware the application is not complete without the required VA
letter. When the application is complete, the assessing official shall inform
the veteran or surviving spouse by mail whether or not the application is
approved, the veteran or surviving spouse is exempt from the real property tax,
and if exempted, the amount of the exemption.
D. The veteran or surviving spouse shall be required to
re-file the application and notify the previous jurisdiction, required by this
section only, if the principal place of residence changes.
E. While there is no deadline to apply for the exemption,
the Commissioner of the Revenue, or assessing official, may only correct and
refund (without interest) the past assessments of an initially qualified
applicant for no more than the current tax year, plus up to three prior tax
years after January 1, 2011.
F. No county, city, or town shall be liable for any
interest on any refund due to the veteran or surviving spouse for taxes paid
prior to the filing of the application required by § 58.1-3219.6 of the
Code of Virginia.
G. In the determination of the exemption, no
locality may implement income or asset limitations or a deadline for
application.
H. This chapter does not prohibit the locality's ability
to require an annual confirmation of continued residence from the qualifying
veteran or surviving spouse.
1VAC80-10-100. Informal requests for information; formal
appeals process.
A. The commissioner will provide written guidance to and
respond to requests for information from Commissioners of the Revenue, other
assessing officials, or veterans regarding the exemption, including
interpretation of the provisions of subdivision (a) of Section 6-A of Article X
of the Constitution of Virginia and the implementing statutes. Such requests
may be by telephone or in writing. Request for an appeal must be in writing.
B. The commissioner does not have the authority to answer
questions regarding the assessed value of any property. Such questions should
be answered solely by the veteran's respective Commissioner of Revenue or other
assessing official.
C. A veteran or surviving spouse desiring to appeal a
denial of an application for exemption by a Commissioner of the Revenue or
other assessing official shall send a written request for appeal and the
document from the veteran's respective Commissioner of Revenue or other
assessing official denying the veteran's application as follows:
1. By electronic mail to john.newby@dvs.virginia.gov; carrieann.alford@dvs.virginia.gov
with a subject line that states "ATTN: Tax Exemption – APPEAL"; or
2. By U.S. mail or delivery to Commissioner, Virginia
Department of Veterans Services, "ATTN: Tax Exemption - APPEAL,"
101 N. 14th Street, 17th Floor, Richmond VA 23219.
D. The commissioner may conduct hearings telephonically,
by video conferencing means, or if the commissioner determines it necessary, in
person at the department's headquarters in Richmond. The appeal shall be
limited to issues involving the tax exemption eligibility criteria. The
commissioner is not authorized to hear or decide appeals regarding a dispute
over a property's assessed value.
E. In advance of any hearing, both the veteran, or
surviving spouse, and the Commissioner of the Revenue, or other assessing
official, shall be provided (i) reasonable notice of the time, date, and
location of the hearing; (ii) the right to appear in person or by counsel, or
other qualified representative, before the agency or its subordinates for the
presentation of factual data, argument, or proof in connection with any case;
and (iii) notice of all facts or information in the possession of the
department that could be relied upon in making a decision.
F. The commissioner shall render a decision within 90 days
from the date of the hearing, or from a later date agreed to by the veteran, or
surviving spouse, and the commissioner. If the commissioner does not render a
decision within 90 days, the veteran may provide written notice to the
commissioner that a decision is due. If no decision is made within 30 days from
the commissioner's receipt of the notice, the decision shall be deemed to be in
favor of the veteran.
G. The final decision by the commissioner shall be mailed
to all named parties.
H. A decision of the commissioner may be appealed by
either party to the circuit court in the locality in which the veteran or
surviving spouse resides.
I. The burden shall be upon the party complaining of the
commissioner's decision to designate and demonstrate an error of law subject to
review by the circuit court. Such issues of law include: (i) accordance with
constitutional right, power, privilege, or immunity; (ii) compliance with
statutory authority, jurisdiction limitations, or right as provided in the
basic laws as to subject matter and the factual showing respecting entitlement
in connection with case decisions; (iii) observance of required procedure where
any failure therein is not mere harmless error; and (iv) the substantiality of
the evidentiary support for findings of fact. Any necessary facts in
controversy shall be determined by the court upon the basis of the agency file,
minutes, and records of its proceedings, augmented, if need be, by the agency
pursuant to order of the court or supplemented by any allowable and necessary
proofs adduced in court, except that the function of the court shall be to
determine only whether the result reached by the agency could reasonably be
said, on all such proofs, to be within the scope of the legal authority of the
agency. The court shall take due account of the presumption of official
regularity, the experience and specialized competence of the agency, and the
purposes of the basic law under which the agency has acted.
VA.R. Doc. No. R17-5203; Filed August 22, 2017, 2:36 p.m.
TITLE 4. CONSERVATION AND NATURAL RESOURCES
BOARD OF GAME AND INLAND FISHERIES
Final Regulation
REGISTRAR'S NOTICE: The
Board of Game and Inland Fisheries is claiming an exemption from the
Administrative Process Act pursuant to § 2.2-4002 A 3 of the Code of Virginia
when promulgating regulations regarding the management of wildlife.
Title of Regulation: 4VAC15-70. Game: Bobcat (amending 4VAC15-70-60).
Statutory Authority: §§ 29.1-103 and 29.1-501 of the
Code of Virginia.
Effective Date: September 1, 2017.
Agency Contact: Jeff Trollinger, Deputy Director, Bureau
of Wildlife Resources, Department of Game and Inland Fisheries, 7870 Villa Park
Drive, Suite 400, Henrico, VA 23228, telephone (804) 367-1134, or email
jeff.trollinger@dgif.virginia.gov.
Summary:
The amendments allow hunting bobcats with the slingbow,
which is a type of bow and arrow.
4VAC15-70-60. Archery hunting with bow and arrow or,
crossbow, or slingbow.
A. Season. It shall be lawful to hunt bobcats with bow and
arrow or, crossbow, or slingbow from the first Saturday in
October through October 31, both dates inclusive.
B. Carrying firearms prohibited. It shall be unlawful to
carry firearms while hunting with bow and arrow or, crossbow,
or slingbow during the special archery seasons.
C. Use of dogs prohibited during the special archery season.
It shall be unlawful to use dogs when hunting with bow and arrow or,
crossbow, or slingbow during any special archery season.
VA.R. Doc. No. R17-5195; Filed August 28, 2017, 2:07 p.m.
TITLE 4. CONSERVATION AND NATURAL RESOURCES
BOARD OF GAME AND INLAND FISHERIES
Final Regulation
REGISTRAR'S NOTICE: The
Board of Game and Inland Fisheries is claiming an exemption from the
Administrative Process Act pursuant to § 2.2-4002 A 3 of the Code of Virginia
when promulgating regulations regarding the management of wildlife.
Title of Regulation: 4VAC15-90. Game: Deer (amending 4VAC15-90-70).
Statutory Authority: §§ 29.1-103 and 29.1-501 of the
Code of Virginia.
Effective Date: September 1, 2017.
Agency Contact: Jeff Trollinger, Deputy Director, Bureau
of Wildlife Resources, Department of Game and Inland Fisheries, 7870 Villa Park
Drive, Suite 400, Henrico, VA 23228, telephone (804) 367-1134, or email
jeff.trollinger@dgif.virginia.gov.
Summary:
The amendment allows a county participating in the urban
archery deer hunting season to exclude geographic areas from the season when
consistent with Department of Game and Inland Fisheries deer management
objectives.
4VAC15-90-70. Archery hunting.
A. It shall be lawful to hunt deer during the early special
archery season with archery equipment or a slingbow from the first Saturday in
October through the Friday prior to the third Monday in November, both dates
inclusive.
B. In addition to the season provided in subsection A of this
section, it shall be lawful to hunt deer during the late special archery season
with archery equipment or a slingbow:
1. From the Sunday following the close of the general firearms
season on deer through the first Saturday in January, both dates inclusive, in
(i) all cities, towns, and counties west of the Blue Ridge Mountains (except
Clarke County and on non-national forest lands in Frederick County); (ii) in
the Counties (including the cities and towns within) of Amherst (west of
Business U.S. 29 from the James River to its intersection with U.S. 29 just
south of the Town of Amherst continuing north on U.S. 29 to the Tye River),
Bedford, Franklin, Henry, Nelson (west of Route 151), and Patrick; (iii) on the
Chester F. Phelps Wildlife Management Area; and (iv) on national forest lands
in Frederick County.
2. From December 1 through the first Saturday in January, both
dates inclusive, in the Cities of Chesapeake, Suffolk (east of the Dismal Swamp
Line), and Virginia Beach.
C. Deer of either sex may be taken full season during the
special archery seasons as provided in subsections A and B of this section
(except on PALS (Public Access Lands) in Dickenson County where it shall be
unlawful to take antlerless deer during the special archery seasons provided
for in subsections A and B of this section).
D. It shall be unlawful to carry firearms while hunting with
archery equipment during the special archery seasons, except that a
muzzleloading gun, as defined in 4VAC15-90-80, may be in the possession of a
properly licensed muzzleloading gun hunter when and where a special archery
deer season overlaps a special muzzleloading deer season.
E. It shall be unlawful to use dogs when hunting with archery
equipment during any special archery season, except that tracking dogs as
described in § 29.1-516.1 of the Code of Virginia may be used.
F. It shall be lawful to hunt antlerless deer during the
special urban archery season with archery equipment or a slingbow from the
first Saturday in September through the Friday prior to the first Saturday in
October, both dates inclusive, and from the Sunday following the first Saturday
in January through the last Sunday in March, both dates inclusive, within the
incorporated limits of any city or town in the Commonwealth (except on national
forest and department-owned lands) and counties with a human population density
of 300 persons per square mile or more (except on national forest and
department-owned lands), provided that its governing body submits by certified
letter to the department prior to April 1, its intent to participate in the
special urban archery season. Any city, town, or county no longer participating
in this season shall submit by certified letter to the department prior to
April 1 notice of its intent not to participate in the special urban archery
season. When consistent with the department's deer management objectives and
subject to the director's approval, a participating county may exclude from
this season a geographic area or areas by submitting a clear description of
such area or areas in a certified letter to the department prior to April 1.
G. It shall be lawful to hunt antlerless deer during the
special antlerless archery season with archery equipment or a slingbow from the
Monday following the last Sunday in March through the last Sunday in April, both
dates inclusive, in the Counties of Arlington, Fairfax, Loudoun, and Prince
William (including the cities and towns within).
VA.R. Doc. No. R17-5196; Filed August 28, 2017, 2:28 p.m.
TITLE 4. CONSERVATION AND NATURAL RESOURCES
BOARD OF GAME AND INLAND FISHERIES
Final Regulation
REGISTRAR'S NOTICE: The
Board of Game and Inland Fisheries is claiming an exemption from the
Administrative Process Act pursuant to § 2.2-4002 A 3 of the Code of
Virginia when promulgating regulations regarding the management of wildlife.
Title of Regulation: 4VAC15-260. Game: Waterfowl and
Waterfowl Blinds (adding 4VAC15-260-116).
Statutory Authority: §§ 29.1-103 and 29.1-501 of
the Code of Virginia.
Effective Date: February 1, 2018.
Agency Contact: Jeff Trollinger, Deputy Director, Bureau
of Wildlife Resources, Department of Game and Inland Fisheries, 7870 Villa Park
Drive, Suite 400, Henrico, VA 23228, telephone (804) 367-1134, or email
jeff.trollinger@dgif.virginia.gov.
Summary:
The amendments prohibit the licensing of nonriparian blinds
in public waters in front of National Park Service land along the north shore
of the York River and York River State Park land along the south shore of the
York River.
4VAC15-260-116. Blinds adjacent to Werowocomoco National
Park and York River State Park.
No licenses shall be issued for stationary waterfowl
blinds in front of Werowocomoco National Park in Purtan Bay and on the York
River between Purtan Island and Barren Point in Gloucester County, and in front
of York River State Park between Taskinas Creek and the eastern boundary of
York River State Park in James City County. These prohibitions shall not alter
the privileges prescribed in §§ 29.1-344 and 29.1-347 of the Code of Virginia
for riparian owners and their lessees and permittees.
VA.R. Doc. No. R17-5074; Filed August 28, 2017, 8:56 a.m.
TITLE 4. CONSERVATION AND NATURAL RESOURCES
MARINE RESOURCES COMMISSION
Final Regulation
REGISTRAR'S NOTICE: The
Marine Resources Commission is claiming an exemption from the Administrative
Process Act in accordance with § 2.2-4006 A 11 of the Code of Virginia;
however, the commission is required to publish the full text of final
regulations.
Title of Regulation: 4VAC20-720. Pertaining to
Restrictions on Oyster Harvest (amending 4VAC20-720-20, 4VAC20-720-40,
4VAC20-720-60, 4VAC20-720-70, 4VAC20-720-75, 4VAC20-720-80, 4VAC20-720-90,
4VAC20-720-100).
Statutory Authority: § 28.2-201 of the Code of Virginia.
Effective Date: October 1, 2017.
Agency Contact: Jennifer Farmer, Regulatory Coordinator,
Marine Resources Commission, 2600 Washington Avenue, 3rd Floor, Newport News,
VA 23607, telephone (757) 247-2248, or email jennifer.farmer@mrc.virginia.gov.
Summary:
The amendments establish the 2017-2018 areas of public
harvest, public oyster harvest seasons, and management measures. Measures
include expanding replenishment activities to increase public oyster
production, transitioning areas that reach market oyster populations high
enough to harvest with less efficient gear to that gear type, expanding areas
not currently in a harvest rotation to rotational areas, and restricting public
harvest in areas that do not or have not received replenishment.
4VAC20-720-20. Definitions.
The following words and terms when used in this chapter shall
have the following meanings unless the context clearly indicates otherwise:
"Aid to navigation" means any public or private day
beacon, lighted channel marker, channel buoy, lighted channel buoy, or
lighthouse that may be at, or adjacent to, any latitude and longitude used in
area descriptions.
"Clean culled oyster" means any oyster taken from
natural public beds, rocks, or shoals that is three inches or greater in shell
length.
"Coan River Area" means that area of the Coan River
inside of Public Grounds 77 and 78 of Northumberland County.
Public Ground 77 of Northumberland County is located near the
mouth of the Coan River, beginning at a point approximately 2,300 feet
northeast of Honest Point and 1,300 feet southwest of Travis Point, said point
being Corner 1, located at Latitude 37° 59.5257207' N., Longitude 76°
27.8810639' W.; thence southwesterly to Corner 2, Latitude 37° 59.3710259' N.,
Longitude 76° 27.9962148' W.; thence southwesterly to Corner 3, Latitude 37°
59.2953830' N., Longitude 76° 28.0468953' W.; thence northwesterly to Corner 4,
Latitude 37° 59.3350863' N., Longitude 76° 28.0968837' W.; thence northeasterly
to Corner 5, Latitude 37° 59.3965161' N., Longitude 76° 28.0287342' W.; thence
northwesterly to Corner 6, Latitude 37° 59.4758507' N., Longitude 76°
28.1112280' W.; thence north-northwesterly to Corner 7, Latitude 37°
59.5079401' N., Longitude 76° 28.1230058' W.; thence northeasterly to Corner 8,
Latitude 37° 59.5579153' N., Longitude 76° 27.9889429' W.; thence southeasterly
to Corner 1, said corner being the point of beginning.
Public Ground 78 of Northumberland County is located near the
mouth of the Coan River, beginning at a point approximately 3,420 feet
southeast of Travis Point and 3,260 feet northwest of Great Point, said point
being Corner 1, located at Latitude 37° 59.4822275' N., Longitude 76°
27.1878637' W.; thence southeasterly to Corner 2, Latitude 37° 59.3824046' N.,
Longitude 76° 27.1088650' W.; thence southwesterly to Corner 3, Latitude 37°
59.2283287' N., Longitude 76° 27.8632901' W.; thence northeasterly to Corner 4,
Latitude 37° 59.4368502' N., Longitude 76° 27.6868001' W.; thence continuing
northeasterly to Corner 5, Latitude 37° 59.5949216' N., Longitude 76°
27.5399436' W.; thence southeasterly to Corner 1, said corner being the point
of beginning.
"Deep Rock Area" means all public grounds and
unassigned grounds, in that area of the Chesapeake Bay near Gwynn Island,
beginning at Cherry Point at the western-most point of the eastern headland of
Kibble Pond located at Latitude 37° 30.9802148' N., Longitude 76° 17.6764393'
W.; thence northeasterly to the Piankatank River, Flashing Green Channel Light
"3", Latitude 37° 32.3671325' N., Longitude 76° 16.7038334' W.;
thence east-southeasterly to the Rappahannock River Entrance Lighted Buoy
G"1R", Latitude 37° 32.2712833' N., Longitude 76° 11.4813666' W.; thence
southwesterly to the southern-most point of Sandy Point, the northern headland
of "The Hole in the Wall", Latitude 37° 28.1475258' N., Longitude 76°
15.8185670' W.; thence northwesterly along the Chesapeake Bay mean low water
line of the barrier islands of Milford Haven, connecting headland to headland
at their eastern-most points, and of Gwynn Island to the western-most point of
the eastern headland of Kibble Pond on Cherry Point, said point being the point
of beginning.
"Deep Water Shoal State Replenishment Seed Area" or
"DWS" means that area in the James River near Mulberry Island,
beginning at a point approximately 530 feet west of Deep Water Shoal Light,
said point being Corner 1, located at Latitude 37° 08.9433287' N., Longitude
76° 38.3213007' W.; thence southeasterly to Corner 2, Latitude 37° 09.5734380'
N., Longitude 76° 37.8300582' W.; thence southwesterly to Corner 3, Latitude
37° 08.9265524' N., Longitude 76° 37.0574269' W.; thence westerly to Corner 4,
Latitude 37° 08.4466039 N., Longitude 76° 37.4523346' W.; thence northwesterly
to Corner 5, Latitude 37° 08.4491489' N., Longitude 76° 38.0215553' W.; thence
northeasterly to Corner 1, said corner being the point of beginning.
"Great Wicomico River Area" means all public
grounds and unassigned grounds, in that area of the Great Wicomico River,
Ingram Bay, and the Chesapeake Bay, beginning at a point on Sandy Point,
Latitude 37° 49.3269652' N., Longitude 76° 18.3821766' W.; thence easterly to
the southern-most point of Cockrell Point, Latitude 37° 49.2664838' N., Longitude
76° 17.3454434' W.; thence easterly following the mean low water line of
Cockrell Point to a point on the boundary of Public Ground 115 at Cash Point,
Latitude 37° 49.2695619' N., Longitude 76° 17.2804046' W.; thence southeasterly
to the gazebo on the pierhead at Fleets Point, Latitude 37° 48.7855824' N.,
Longitude 76° 16.9609311' W.; thence southeasterly to the Great Wicomico
Lighthouse; thence due south to a point due east of the southern-most point of
Dameron Marsh, Latitude 37° 46.6610003' N., Longitude 76° 16.0570007' W.;
thence due west to the southern-most point of Dameron Marsh, Latitude 37°
46.6609070' N., Longitude 76° 17.2670707' W.; thence along the mean low water
line of Dameron Marsh, north and west to Garden Point, Latitude 37° 47.2519872'
N., Longitude 76° 18.4028142' W.; thence northwesterly to Windmill Point,
Latitude 37° 47.5194547' N., Longitude 76° 18.7132194' W.; thence northerly
along the mean low water to the western headland of Harveys Creek, Latitude 37°
47.7923573' N., Longitude 76° 18.6881450' W.; thence east-southeasterly to the
eastern headland of Harveys Creek, Latitude 37° 47.7826936' N., Longitude 76°
18.5469879' W.; thence northerly along the mean low water line, crossing the
entrance to Towels Creek at the offshore ends of the jetties and continuing to
Bussel Point, Latitude 37° 48.6879208' N., Longitude 76° 18.4670860' W.; thence
northwesterly to the northern headland of Cranes Creek, Latitude 37°
48.8329168' N., Longitude 76° 18.7308073' W.; thence following the mean low
water line northerly to a point on Sandy Point, said point being the point of
beginning.
"Great Wicomico River Rotation Area 1"
means all public grounds and unassigned grounds, in that area of the Great
Wicomico River, Ingram Bay, and the Chesapeake Bay, beginning at a point on
Sandy Point, Latitude 37° 49.3269652' N., Longitude 76° 18.3821766' W.; thence
easterly to the southern-most point of Cockrell Point, Latitude 37° 49.2664838'
N., Longitude 76° 17.3454434' W.; thence easterly following the mean low water
line of Cockrell Point to a point on the boundary of Public Ground 115 at Cash
Point, Latitude 37° 49.2695619' N., Longitude 76° 17.2804046' W.; thence
southeasterly to the gazebo on the pier head at Fleeton Point, Latitude 37°
48.7855824' N., Longitude 76° 16.9609311' W.; thence southeasterly to the Great
Wicomico River Light; Latitude 37° 48.2078167' N., Longitude 76° 15.9799333'
W.; thence westerly to a point on the offshore end of the southern jetty at the
entrance to Towles Creek, Latitude 37° 48.3743771' N., Longitude 76° 17.9600320
W.; thence northerly crossing the entrance to Towles Creek at the offshore ends
of the jetties and continuing along the mean low water line to Bussel Point,
Latitude 37° 48.6879208' N., Longitude 76° 18.4670860' W.; thence northwesterly
to the northern headland of Cranes Creek, Latitude 37° 48.8329168' N.,
Longitude 76° 18.7308073' W.; thence following the mean low water line
northerly to a point on Sandy Point, Latitude 37° 49.3269652' N., Longitude 76°
18.3821766' W., said point being the point of beginning.
"Great Wicomico River Rotation Area 2" means all
public grounds and unassigned grounds, in that area of the Great Wicomico
River, Ingram Bay, and the Chesapeake Bay, beginning at a point on Great
Wicomico River Light, Latitude 37° 48.2078167' N., Longitude 76° 15.9799333'
W.; thence due south to a point due east of the southern-most point of Dameron
Marsh, Latitude 37° 46.6610003' N., Longitude 76° 16.0570007' W.; thence due
west to the southern-most point of Dameron Marsh, Latitude 37° 46.6609070' N.,
Longitude 76° 17.2670707' W.; thence along the mean low water line of Dameron
Marsh, north and west to Garden Point, Latitude 37° 47.2519872' N., Longitude
76° 18.4028142' W.; thence northwesterly to Windmill Point, Latitude 37°
47.5194547' N., Longitude 76° 18.7132194' W.; thence northerly along the mean
low water line to the western headland of Harveys Creek, Latitude 37°
47.7923573' N., Longitude 76° 18.6881450' W.; thence east-southeasterly to the
eastern headland of Harveys Creek, Latitude 37° 47.7826936' N., Longitude 76°
18.5469879' W.; thence northerly along the mean low water line to a point on
the offshore end of the southern jetty at the entrance to Towles Creek,
Latitude 37° 48.3743771' N., Longitude 76° 17.9600320' W., thence easterly to
Great Wicomico River Light, Latitude 37° 48.2078167' N., Longitude 76°
15.9799333' W., said point being the point of beginning.
"Hand scrape" means any device or instrument with a
catching bar having an inside measurement of no more than 22 inches, which is
used or usable for the purpose of extracting or removing shellfish from a water
bottom or the bed of a body of water.
"Hand tong" or "ordinary tong" means any
pincers, nippers, tongs, or similar device used in catching oysters, which
consist of two shafts or handles attached to opposable and complementary
pincers, baskets, or containers operated entirely by hand, from the surface of
the water and has no external or internal power source.
"James River Area" means those public grounds of
the James River and Nansemond River west of the Monitor Merrimac Memorial
Bridge Tunnel (Route I-664), northeast of the Mills E. Godwin, Jr. Bridge (U.S.
Route 17) on the Nansemond River, and south of the James River Bridge (U.S.
Route 17).
"James River Hand Scrape Area 1" means
all public grounds and unassigned grounds, in that area of the James River,
beginning at the Flashing Green Channel Light #5, located at Latitude 37°
02.3528833' N., Longitude 76° 32.7785333' W.; thence southeasterly to the
Flashing Green Channel Light #3, located at Latitude 37° 01.7124500' N.,
Longitude 76° 31.8210667' W.; thence southeasterly to the Flashing Green
Channel Light #1, located at Latitude 37° 00.7666667' N., Longitude 76°
29.9083333' W.; thence southeasterly to the northeast corner of the western
draw span pier of the James River Bridge (U.S. Route 17), Latitude 37°
00.1524824' N., Longitude 76° 28.1581984' W.; thence southwesterly along the
upstream side of the James River Bridge to the mean low water line; thence
northwesterly along the mean low water line, crossing Kings Creek at the
headlands and continuing along the mean low water line to a point on the shore
at Rainbow Farm Point in line with VMRC Markers "STH" and
"SMT," located at Latitude 37° 00.1965862' N., Longitude 76°
34.0712010' W.; thence north-northeasterly to a VMRC Marker "STH,"
Latitude 37° 00.9815328 N., Longitude 76° 33.5955842' W.; thence to a VMRC
Marker "SMT," at Latitude 37° 01.3228160' N., Longitude 76° 33.3887351'
W.; thence to the Flashing Green Channel Light #5, at Latitude 37° 02.3528833'
N., Longitude 76° 32.7785333' W., said point being the point of beginning.
"James River Hand Scrape Area 2" means
all public grounds and unassigned grounds, in that area of the James River, beginning
at the Flashing Green Channel Light #5, located at Latitude 37° 02.3528833' N.,
Longitude 76° 32.7785333' W.; thence northeasterly to a VMRC Marker
"NMT," Latitude 37° 02.7740540' N., Longitude 76° 32.0960864' W.;
thence to a VMRC Marker "NTH" located at Latitude 37° 03.2030055' N.,
Longitude 76° 31.4231211' W.; thence to a point on the north shore of the river
at Blunt (Blount) Point, said point being in line with VMRC Markers
"NMT" and "NTH" and located at Latitude 37° 03.3805862' N.,
Longitude 76° 31.1444562' W.; thence southeasterly along the mean low water
line to the upstream side of the James River Bridge (U.S. Route 17); thence
westerly along the James River Bridge to the northeast corner of the western
draw span pier, Latitude 37° 00.1524824' N., Longitude 76° 28.1581984' W.;
thence northwesterly to the Flashing Green Channel Light #1, located at
Latitude 37° 00.7666667' N., Longitude 76° 29.9083333' W.; thence northwesterly
to the Flashing Green Channel Light #3, located at Latitude 37° 01.7124500' N.,
Longitude 76° 31.8210667' W.; thence northwesterly to the Flashing Green
Channel Light #5, located at Latitude 37° 02.3528833' N., Longitude 76°
32.7785333' W., said point being the point of beginning
"James River Hand Scrape Area 3" means those
public grounds of Isle of Wight County and Nansemond County (City of Suffolk)
located in the James River and Nansemond River west of the Monitor Merrimac
Memorial Bridge Tunnel (Route I-664), northeast of the Mills E. Godwin, Jr.
Bridge (U.S. Route 17) on the Nansemond River, and south of the James River
Bridge (U.S. Route 17).
"James River Seed Area" means all public
grounds and unassigned grounds in that area of the James River and its
tributaries with a southeastern boundary beginning at a point on the shore on
the south side of the river at Rainbow Farm Point in Isle of Wight County
located at Latitude 37° 00.1965862' N., Longitude 76° 34.0712010' W.; thence
north-northeasterly to a VMRC Marker "STH," Latitude 37° 00.9815328
N., Longitude 76° 33.5955842' W.; thence to a VMRC Marker "SMT," at
Latitude 37° 01.3228160' N., Longitude 76° 33.3887351' W.; thence to the
Flashing Green Channel Light #5, at Latitude 37° 02.3449949' 02.3528833'
N., Longitude 76° 32.7689936' 32.7785333' W.; thence
northeasterly to a VMRC Marker "NMT," Latitude 37° 02.7740540' N.,
Longitude 76° 32.0960864' W.; thence to a VMRC Marker "NTH" located
at Latitude 37° 03.2030055' N., Longitude 76° 31.4231211' W.; thence to a point
on the north shore of the river at Blunt (Blount) Point, in the City of Newport
News, located at Latitude 37° 03.3805862' N., Longitude 76° 31.1444562' W.; the
northern boundary, being a straight line, beginning at a point on the shore on
the east side of the river in the City of Newport News, at Latitude 37° 08.4458787'
N., Longitude 76° 37.2855533' W.; thence westerly to the southeast corner of
the Deep Water Shoal State Replenishment Seed Area, Latitude 37° 08.4466039'
N., Longitude 76° 37.4523346' W.; thence westerly to the southwest corner of
the Deep Water Shoal State Replenishment Seed Area, Latitude 37° 08.4490472'
N., Longitude 76° 38.0215554' W.; thence westerly to a point on the shore on
the west side of the river at the mouth of Lawnes Creek in Isle of Wight
County, Latitude 37° 08.4582990' N., Longitude 76° 40.2816023' W.
"Latitude and longitude" means values that are
based upon a geodetic reference system of the North American Datum of 1983
(NAD83). When latitude and longitude are used in any area description, in
conjunction with any physical landmark, to include aids to navigation, the
latitude and longitude value is the legal point defining the boundary.
"Little Wicomico River" means that area of the
Little Wicomico River inside of Public Ground 43 of Northumberland County,
located in the Little Wicomico River near Bridge Creek, beginning at a point
approximately 150 feet north of Peachtree Point, said point being Corner 1,
located at Latitude 37° 53.2910650' N., Longitude 76° 16.7312926' W.; thence
southwesterly to Corner 2, Latitude 37° 53.2601877' N., Longitude 76°
16.8662408' W.; thence northwesterly to Corner 3, Latitude 37° 53.2678470' N.,
Longitude 76°16.8902408' W.; thence northeasterly to Corner 4, Latitude 37°
53.3113148' N., Longitude 76° 16.8211543' W.; thence southeasterly to Corner 1,
said corner being the point of beginning.
"Milford Haven" means that area of Milford Haven
inside of Public Ground 7 of Mathews County, beginning at a point approximately
1,380 feet east of Point Breeze, said point being Corner 1, located at Latitude
37° 28.3500000' N., Longitude 76° 16.5000000' W.; thence northeasterly to
Corner 2, Latitude 37° 28.3700000' N., Longitude 76° 16.4700000' W.; thence
southeasterly to Corner 3, Latitude 37° 28.3500000' N., Longitude 76°
16.4200000' W.; thence southwesterly to Corner 4, Latitude 37° 28.3200000' N.,
Longitude 76° 16.4500000' W.; thence northwesterly to Corner 1, said corner
being the point of beginning.
"Mobjack Bay Area" means that area of Mobjack Bay
consisting of Public Ground 25 2 of Gloucester Mathews
County (Tow Stake) (Pultz Bar) described as:
Public Ground 25 of Gloucester County, known as Tow Stake,
is located in Mobjack Bay, near the mouth of the Severn River, beginning at a
point approximately 2,880 feet east-northeast of Tow Stake Point, said point
being Corner 1, located at Latitude 37° 20.3883888' N., Longitude 76°
23.5883836' W.; thence northeasterly to Corner 2, Latitude 37° 30.5910482' N.,
Longitude 76° 23.2372184' W.; thence southeasterly to Corner 3, Latitude 37°
20.3786971' N., Longitude 76° 22.7241180' W.; thence southwesterly to Corner 4,
Latitude 37° 19.8616759' N., Longitude 76° 23.5914937' W.; thence northwesterly
to Corner 5, Latitude 37° 20.0284019' N., Longitude 76° 23.7717423' W.; thence
northeasterly to Corner 1, said corner being the point of beginning.
Public Ground 2 of Mathews County, known as Pultz Bar, is
located in Mobjack Bay, beginning at a point approximately 5,420 feet south of
Minter Point, said point being Corner 1, located at Latitude 37°
21.2500000' N., Longitude 76° 21.3700000' W.; thence easterly to Corner 2,
Latitude 37° 21.2700000' N., Longitude 76° 20.9600000' W.; thence southerly to
Corner 3, Latitude 37° 21.0200000' N., Longitude 76° 20.9400000' W.; thence
westerly to Corner 4, Latitude 37° 21.0500000' N., Longitude 76° 21.3300000'
W.; thence northerly to Corner 1, said corner being the point of beginning.
"Nomini Creek Area" means that area of Nomini Creek
inside of Public Grounds 26 and 28 of Westmoreland County.
Public Ground 26 of Westmoreland County is located in Nomini
Creek, north of Beales Wharf and east of Barnes Point, beginning at a point
approximately 1,400 feet north of Barnes Point, said point being Corner 1,
located at Latitude 38° 07.2690219' N., Longitude 76° 42.6784210' W.; thence
southeasterly to Corner 2, Latitude 38° 07.0924060' N., Longitude 76°
42.4745767' W.; thence southwesterly to Corner 3, Latitude 38° 06.8394053' N.,
Longitude 76° 42.6704025, W.; thence northwesterly to Corner 4, Latitude 38°
06.8743004' N., Longitude 76° 42.7552151' W.; thence northeasterly to Corner 5,
Latitude 38° 07.0569717' N., Longitude 76° 42.5603535' W.; thence northwesterly
to Corner 1, said corner being the point of beginning.
Public Ground 28 of Westmoreland County is located at the
mouth of Nomini Creek, beginning at a point approximately 50 feet west of White
Oak Point, said point being Corner 1, located at Latitude 38° 07.6429987' N.,
Longitude 76° 43.0337082' W.; thence south-southeasterly to Corner 2, Latitude
38° 07.2987193' N., Longitude 76° 43.1101420' W.; thence northwesterly to
Corner 3, Latitude 38° 07.7029267' N., Longitude 76° 43.3337762' W.; thence
west to the mean low water line, Latitude 38° 07.7031535' N., Longitude 76°
43.3378345' W.; thence northerly and westerly along the mean low water line of
Nomini Creek to a point southwest of Cedar Island, Latitude 38° 07.8986449' N.,
Longitude 76° 43.6329097' W.; thence northeasterly to a point on the mean low
water line at the southern-most point of Cedar Island, Latitude 38° 07.8986449'
N., Longitude 76° 43.6329097' W.; thence following the mean low water line of
the southern and eastern sides of Cedar Island to a point, Latitude 38°
08.0164430' N., Longitude 76° 43.4773169' W.; thence northeasterly to Corner 4,
Latitude 38° 08.0712849' N., Longitude 76° 43.4416606' W.; thence northeasterly
to a point on the northern headland of Nomini Creek at the mean low water line,
said point being Corner 5, Latitude 38° 08.2729626' N., Longitude 76°
43.3105315' W.; thence following the mean low water line of White Point to a
point northwest of Snake Island, Corner 6, Latitude 38° 08.4066960' N.,
Longitude 76° 42.9105565' W.; thence southeast, crossing the mouth of Buckner
Creek, to a point on the mean low water line of Snake Island, Corner 7,
Latitude 38° 08.3698254' N., Longitude 76° 42.8939656' W.; thence southeasterly
following the mean low water line of Snake Island to Corner 8, Latitude 38°
08.2333798' N., Longitude 76° 42.7778877' W.; thence south-southwesterly,
crossing the mouth of Buckner Creek, to Corner 9, Latitude 38° 08.2134371' N.,
Longitude 76° 42.7886409' W.; thence southeasterly to a point on the mean low
water line of the southern headland of Buckner Creek, Corner 10, Latitude 38°
08.1956281' N., Longitude 76° 42.7679625' W.; thence southwesterly following the
mean low water line of Nomini Creek, crossing the mouth of an un-named cove at
the narrowest point between the headlands and continuing to follow the mean low
water line to a point on White Oak Point, Latitude 38° 07.6428228' N.,
Longitude 76° 43.0233530' W.; thence west to Corner 1, said point being the
point of beginning.
"Oyster" means any shellfish of the species
Crassostrea virginica.
"Oyster dredge" means any device having a maximum
weight of 150 pounds with attachments, maximum width of 50 inches, and maximum
tooth length of four inches.
"Oyster patent tong" means any patent tong not
exceeding 100 pounds in gross weight, including any attachment other than rope
and with the teeth not to exceed four inches in length.
"Oyster resource user fee" means a fee that must be
paid each calendar year by anyone who grows, harvests, shucks, packs, or ships
oysters for commercial purposes.
"Pocomoke Sound Area" means that area of Pocomoke
Sound inside of Public Grounds 9 and 10 of Accomack County.
Public Ground 9 of Accomack County is located in the Pocomoke
Sound, beginning at a corner on the Maryland-Virginia state line, located in
the Pocomoke Sound approximately 1.06 nautical miles north-northeast of the
northern-most point of North End Point, said point being Corner 1, located at
Latitude 37° 57.2711566' N., Longitude 75° 42.2870790' W. (NAD83); thence
east-northeasterly along the Maryland-Virginia state line to Corner 2, Latitude
37° 57.2896577' N., Longitude 75° 41.9790727' W.; thence southerly to Corner 3,
Latitude 37° 57.2574850' N., Longitude 75° 41.9790730' W.; thence southwesterly
to Corner 4, Latitude 37° 57.2288700' N., Longitude 75° 42.0077287' W.; thence
west-southwesterly to Corner 5, Latitude 37° 57.2034533' N., Longitude 75°
42.1511250' W.; thence south-southwesterly to Corner 6, Latitude 37°
57.0940590' N., Longitude 75° 42.1935214' W.; thence south-southeasterly to
Corner 7, Latitude 37° 57.0551726' N., Longitude 75° 42.1814457' W.; thence
southwesterly to Corner 8, Latitude 37° 56.9408327' N., Longitude 75°
42.2957912' W.; thence south-southwesterly to Corner 9, Latitude 37°
56.6574947' N., Longitude 75° 42.3790819' W.; thence southwesterly to Corner
10, Latitude 37° 56.5790952' N., Longitude 75° 42.5228752' W.; thence
west-southwesterly to Corner 11, Latitude 37° 56.5712564' N., Longitude 75°
42.5915437' W.; thence south-southeasterly to Corner 12, Latitude 37°
56.5441067' N., Longitude 75° 42.5869894' W.; thence southwesterly to Corner
13, Latitude 37° 56.4575045' N., Longitude 75° 42.7458050' W.; thence
west-southwesterly to Corner 14, Latitude 37° 56.2575123' N., Longitude 75°
43.3791097' W.; thence southwesterly to Corner 15, Latitude 37° 55.7408688' N.,
Longitude 75° 43.7957804' W.; thence westerly to Corner 16, Latitude 37°
55.7575327' N., Longitude 75° 43.9458298' W.; thence northwesterly to Corner
17, Latitude 37° 55.8908661' N., Longitude 75° 44.1291309' W.; thence
north-northeasterly to Corner 18, Latitude 37° 55.9908639' N., Longitude 75°
44.0791266' W.; thence northeasterly to Corner 19, Latitude 37° 56.1241858' N.,
Longitude 75° 43.8791328' W.; thence north-northeasterly to Corner 20, Latitude
37° 56.4075136' N., Longitude 75° 43.7291361' W.; thence northeasterly to
Corner 21, Latitude 37° 56.8241664' N., Longitude 75° 43.2624601' W.; thence
north-northeasterly to Corner 22, Latitude 37° 57.0706006' N., Longitude 75°
43.1480402' W.; thence east-northeasterly along the Maryland-Virginia state
line to Corner 1, said corner being the point of beginning.
Public Ground 10 of Accomack County is located in the Pocomoke
Sound, beginning at a corner on the Maryland-Virginia state line, located in
the Pocomoke Sound approximately 2.3 nautical miles westerly of the
northern-most point of North End Point, said point being Corner 1, located at
Latitude 37° 56.4741881' N., Longitude 75° 45.7051676' W. (NAD83); thence
east-northeasterly along the Maryland-Virginia state line to Corner 2, Latitude
37° 56.9261140' N., Longitude 75° 43.7679786' W.; thence south-southwesterly to
Corner 3, Latitude 37° 56.1241948' N., Longitude 75° 44.3624962' W.; thence
west-southwesterly to Corner 4, Latitude 37° 56.0820561' N., Longitude 75°
44.5826292' W.; thence northerly to Corner 5, Latitude 37° 56.1377309' N.,
Longitude 75° 44.5817745' W.; thence west-southwesterly to Corner 6, Latitude
37° 56.1259751' N., Longitude 75° 44.6226859' W.; thence southwesterly to
Corner 7, Latitude 37° 56.1039335' N., Longitude 75° 44.6692334' W.; thence
southerly to Corner 8, Latitude 37° 56.0643616' N., Longitude 75° 44.6750106'
W.; thence west-southwesterly to Corner 9, Latitude 37° 55.9742005' N.,
Longitude 75° 45.1458109' W.; thence west-northwesterly to Corner 10, Latitude
37° 56.0741973' N., Longitude 75° 45.8958329' W.; thence north-northwesterly to
Corner 11, Latitude 37° 56.2565760' N., Longitude 75° 46.0000557' W.; thence
northeasterly along the Maryland-Virginia state line to Corner 1, said corner
being the point of beginning.
"Pocomoke and Tangier Sounds Management Area" or
"PTSMA" means the area as defined in § 28.2-524 of the Code of
Virginia.
"Pocomoke and Tangier Sounds Rotation Area 1" means
all public grounds and unassigned grounds, within an area of the PTSMA, in
Pocomoke and Tangier Sounds, bounded by a line beginning at a point on the
Maryland-Virginia state line, located at Latitude 37° 54.6136000' N., Longitude
75° 53.9739600' W.; thence south to the house on Great Fox Island, Latitude 37°
53.6946500' N., Longitude 75° 53.8898800' W.; thence westerly to a point,
Latitude 37° 53.3633500' N., Longitude 75° 56.5589600' W.; thence south to a
point, Latitude 37° 48.4429100' N., Longitude 75° 56.4883600' W.; thence
easterly to the north end of Watts Island, Latitude 37° 48.7757800' N.,
Longitude 75° 53.5994100' W.; thence northerly to the house on Great Fox
Island, Latitude 37° 53.6946500' N., Longitude 75° 53.8898800' W.; thence
southeasterly to Pocomoke Sound Shoal Flashing Light Red "8",
Latitude 37° 52.4583300' N., Longitude 75° 49.4000000' W.; thence southeasterly
to Messongo Creek Entrance Buoy Green Can "1", Latitude 37°
52.1000000' N., Longitude 75° 47.8083300' W.; thence southeast to Guilford
Flats Junction Light Flashing 2+1 Red "GF", Latitude 37° 50.9533300'
N., Longitude 75° 46.6416700' W.; thence southerly to a point on a line from
Guilford Flats Junction Light to the northern-most point of Russell Island,
where said line intersects the PTSMA boundary, Latitude 37° 48.4715943' N.,
Longitude 75° 46.9955932' W.; thence clockwise following the PTSMA boundary to
a point on the Maryland-Virginia state line, said point being the point of
beginning.
"Pocomoke and Tangier Sounds Rotation Area 2" means
all public grounds and unassigned grounds, within an area of the PTSMA, in
Pocomoke and Tangier Sounds, bounded by a line beginning at the house on Great
Fox Island, located at Latitude 37° 53.6946500' N., Longitude 75° 53.8898800'
W.; thence southerly to the north end of Watts Island, Latitude 37° 48.7757800'
N., Longitude 75° 53.5994100' W.; thence westerly to a point, Latitude 37°
48.4429100' N., Longitude 75° 56.4883600' W.; thence northerly to a point,
Latitude 37° 53.3633500' N., Longitude 75° 56.5589600' W.; thence easterly to
the house on Great Fox Island, said house being the point of beginning. Also,
Pocomoke and Tangier Sounds Rotation Area 2 shall include all public grounds
and unassigned grounds in the PTSMA in Pocomoke Sound bounded by a line
beginning at a point on the Maryland-Virginia state line, Latitude 37°
54.6136000' N., Longitude 75° 53.9739600' W.; thence following the PTSMA
boundary clockwise to a point on the line from the northern-most point of
Russell Island to Guilford Flats Junction Light Flashing 2+1 Red
"GF", where said line intersects the PTSMA boundary, Latitude 37°
48.4715943' N., Longitude 75° 46.9955932' W.; thence northerly to Guilford
Flats Junction Light Flashing 2+1 Red "GF", Latitude 37° 50.9533300'
N., Longitude 75° 46.6416700' W.; thence northwesterly to Messongo Creek
Entrance Buoy Green Can "1", Latitude 37° 52.1000000' N., Longitude
75° 47.8083300' W.; thence northwesterly to Pocomoke Sound Shoal Flashing Light
Red "8", Latitude 37° 52.4583300' N., Longitude 75° 49.4000000' W.;
thence northwesterly to the house on Great Fox Island, Latitude 37° 53.6946500'
N., Longitude 75° 53.8898800' W.; thence northerly to a point on the Maryland-Virginia
state line, said point being the point of beginning.
"Public oyster ground" means all those grounds
defined in § 28.2-551 of the Code of Virginia or by any other acts of the
General Assembly pertaining to those grounds, all those grounds set aside by court
order, and all those grounds set aside by order of the Marine Resources
Commission, and may be redefined by any of these legal authorities.
"Rappahannock River Area 7" means all public
grounds, in that area of the Rappahannock River, bounded downstream by a line
from Rogue Point, located at Latitude 37° 40.0400000' N., Longitude 76°
32.2530000' W.; thence west-northwesterly to Flashing Red Buoy "8",
Latitude 37° 40.1580000' N., Longitude 76° 32.9390000' W.; thence southwesterly
to Balls Point, Latitude 37° 39.3550000' N., Longitude 76° 34.4440000' W.; and
bounded upstream by a line from Punchbowl Point, Latitude 37° 44.6750000' N.,
Longitude 76° 37.3250000' W.; thence southeasterly to Monaskon Point, Latitude
37° 44.0630000' N., Longitude 76° 34.1080000' W.
"Rappahannock River Area 8" means all public
grounds, in that area of the Rappahannock River, bounded downstream by a line
from Monaskon Point, located at Latitude 37° 44.0630000' N., Longitude 76°
34.1080000' W.; thence northwesterly to Punchbowl Point, Latitude 37°
44.6750000' N., Longitude 76° 37.3250000' W.; and bounded upstream by a line
from Jones Point, Latitude 37° 46.7860000' N., Longitude 76° 40.8350000' W.;
thence north-northwesterly to Sharps Point, Latitude 37° 49.3640000' N.,
Longitude 76° 42.0870000' W.
"Rappahannock River Area 9" means all public
grounds, in that area of the Rappahannock River, bounded downstream by a line
from Sharps Point, located at Latitude 37° 49.3640000' N., Longitude 76°
42.0870000' W.; thence south-southeasterly to Jones Point, Latitude 37°
46.7860000' N., Longitude 76° 40.8350000' W.; and bounded upstream by the
Thomas J. Downing Bridge (U.S. Route 360).
"Rappahannock River Rotation Area 1" means
all public grounds, in that area of the Rappahannock River and Chesapeake Bay,
bounded by a line offshore and across the mouth of the Rappahannock River from
a point on the mean low water line of Windmill Point, located at Latitude
37° 36.8200000' N., Longitude 76° 16.9460000' W.; thence southeast to Windmill
Point Light, Latitude 37° 35.7930000' N., Longitude 76° 14.1800000' W.; thence
southwesterly to Stingray Point Light, Latitude 37° 33.6730000' N., Longitude
76° 16.3620000' W.; thence westerly to a point on the mean low water line of
Stingray Point, Latitude 37° 33.6920000' N., Longitude 76° 17.9860000' W.; and
bounded upstream by a line from the mean low water line west of Broad Creek,
Latitude 37° 33.9520000' N., Longitude 76° 19.3090000' W.; thence northeasterly
to a VMRC Buoy on the Baylor line, Latitude 37° 34.5310000' N., Longitude 76°
19.1430000' W.; thence northeasterly to a VMRC Buoy, Latitude 37° 34.6830000'
N., Longitude 76° 19.1000000' W.; thence northwesterly to a VMRC Buoy, Latitude
37° 35.0170000' N., Longitude 76° 19.4500000' W.; thence northwesterly to
Sturgeon Bar Light "7R", Latitude 37° 35.1500000' N., Longitude 76°
19.7330000' W.; thence continuing northwesterly to Mosquito Point Light
"8R", Latitude 37° 36.1000000' N., Longitude 76° 21.3000000' W.;
thence northwesterly to the southern-most corner of the house on Mosquito
Point, Latitude 37° 36.5230000' N., Longitude 76° 21.5950000' W.
"Rappahannock River Rotation Area 2" means all
public grounds, in that area of the Rappahannock River, bounded downstream by a
line from the southern-most corner of the house on Mosquito Point, located at
Latitude 37° 36.5230000' N., Longitude 76° 21.5950000' W.; thence southeast to
Mosquito Point Light "8R", Latitude 37° 36.1000000' N., Longitude 76°
21.3000000' W.; thence continuing southeasterly to Sturgeon Bar Beacon
"7R", Latitude 37° 35.1500000' N., Longitude 76° 19.7330000' W.;
thence west-southwesterly to a VMRC Buoy, Latitude 37° 34.9330000' N.,
Longitude 76° 21.0500000' W.; thence southwesterly to a VMRC Buoy, Latitude 37°
34.8830000' N., Longitude 76° 21.1000000' W.; thence southwesterly to a pier
west of Hunting Creek at Grinels, Latitude 37° 34.4360000' N., Longitude 76°
26.2880000' W.; and bounded on the upstream by a line from Mill Creek Channel
Marker "4", Latitude 37° 35.0830000' N., Longitude 76° 26.9500000'
W.; thence northeasterly to Mill Creek Channel Marker "2", Latitude
37° 35.4830000' N., Longitude 76° 24.5670000' W.; thence northeasterly to the
southern-most corner of the house on Mosquito Point, Latitude 37° 36.5230000'
N., Longitude 76° 21.5950000'0 W.
"Rappahannock River Rotation Area 3" means all
public grounds, in that area of the Rappahannock River, beginning from the
north channel fender at the Robert O. Norris, Jr. Bridge, located at Latitude
37° 37.4830000' N., Longitude 76° 25.3450000' W.; thence southeast to the
southern-most corner of the house on Mosquito Point, Latitude 37° 36.5230000'
N., Longitude 76° 21.5950000' W.; thence southwest to Mill Creek Channel Marker
"2", Latitude 37° 35.4830000' N., Longitude 76° 24.5670000' W.;
thence southwesterly to Mill Creek Channel Marker "4", Latitude 37°
35.0830000' N., Longitude 76° 24.9500000' W.; thence northeasterly to Parrotts
Creek Channel Marker "1", Latitude 37° 36.0330000' N., Longitude 76°
25.4170000' W.; thence northerly to VMRC Buoy, Latitude 37° 36.3330000' N.,
Longitude 76° 25.2000000' W.; thence northerly to the north channel fender of
the Robert O. Norris, Jr. Bridge, said point being the point of beginning.
"Rappahannock River Rotation Area 4" means all
public grounds, in that area of the Rappahannock River, Corrotoman River and
Carter Creek, beginning at the White Stone end of the Robert O. Norris, Jr.
Bridge (State Route 3), located at Latitude 37° 38.1290000' N., Longitude 76°
24.7220000' W.; thence along said bridge to the north channel fender, Latitude
37° 37.4830000' N., Longitude 76° 25.3450000' W.; thence westerly to the VMRC
Buoy "5-4", Latitude 37° 38.0050000' N., Longitude 76° 30.0280000'
W.; thence northerly to Old House Point, Latitude 37° 39.1390000' N., Longitude
76° 29.6850000' W.; thence northeasterly to Ball Point, Latitude 37°
41.6600000' N., Longitude 76° 28.6320000' W.; thence southeasterly to VMRC reef
marker "Ferry Bar – North", Latitude 37° 40.3000000' N., Longitude
76° 28.5000000' W.; thence southwesterly to VMRC reef marker "Ferry Bar –
South", Latitude 37° 40.1670000' N., Longitude 76° 28.5830000' W.; thence
southeasterly to a duck blind west of Corrotoman Point, Latitude 37°
39.8760000' N., Longitude 76° 28.4200000' W.; thence southerly to VMRC Buoy
"543", Latitude 37° 39.2670000' N., Longitude 76° 27.8500000' W.;
thence southerly to VMRC Buoy "Drumming-West", Latitude 37°
38.8830000' N., Longitude 76° 27.6830000' W.; thence southerly to VMRC Buoy
"Drumming-East", Latitude 37° 38.8330000' N., Longitude 76° 27.5670000'
W.; thence northeasterly to Orchard Point, Latitude 37° 38.9240000' N.,
Longitude 76° 27.1260000' W.
"Rappahannock River Rotation Area 5" means all
public grounds, in that area of the Rappahannock River, beginning at the Greys
Point end of the Robert O. Norris, Jr. Bridge (State Route 3), located at
Latitude 37° 36.8330000' N., Longitude 76° 25.9990000' W.; thence northeasterly
along the bridge to the north channel fender, Latitude 37° 37.4830000' N.,
Longitude 76° 25.3450000' W.; thence west-northwesterly to VMRC Buoy
"5-4", Latitude 37° 38.0050000' N., Longitude 76° 30.0280000' W.;
thence westerly to Buoy "R6", Latitude 37° 38.0330000' N., Longitude
76° 30.2830000' W.; thence south to the eastern headland of Whiting Creek, Latitude
37° 36.6580000' N., Longitude 76° 30.3120000' W.
"Rappahannock River Rotation Area 6" means all
public grounds, in that area of the Rappahannock River, beginning on the
eastern headland of Whiting Creek, located at Latitude 37° 36.6580000' N.,
Longitude 76° 30.3120000' W.; thence north to Buoy "R6", Latitude 37°
38.0330000' N., Longitude 76° 30.2830000' W.; thence northwesterly to VMRC
White House Sanctuary Buoy, Latitude 37° 38.1500000' N., Longitude 76°
30.5330000' W.; thence northwesterly to VMRC Towles Point Area Buoy, Latitude
37° 38.8330000' N., Longitude 76° 31.5360000' W.; thence northwesterly to
Flashing Red Buoy "8" off Rogue Point, Latitude 37° 40.1580000' N.,
Longitude 76° 32.9390000' W.; thence southwesterly to Balls Point, Latitude 37°
39.3550000' N., Longitude 76° 34.4440000' W.
"Seed oyster" means any oyster taken by any person
from natural beds, rocks, or shoals that is more than 30 days from harvest for
human consumption.
"Thomas Rock Area" means all public grounds and
unassigned grounds, in that area of the James River, with an eastern boundary
being the upstream side of the James River Bridge (U.S. Route 17), and a
western boundary being a line drawn from the south side of the river at Rainbow
Farm Point, a point on the shore, in line with VMRC Markers "STH" and
"SMT", located at Latitude 37° 00.1965862' N., Longitude 76°
34.0712010' W.; thence north-northeasterly to a VMRC Marker "STH",
Latitude 37° 00.9815328 N., Longitude 76° 33.5955842' W.; thence to a VMRC
Marker "SMT", at Latitude 37° 01.3228160' N., Longitude 76°
33.3887351' W.; thence to the Flashing Green Channel Light #5, at Latitude 37°
02.3449949' N., Longitude 76° 32.7689936' W.; thence northeasterly to a VMRC
Marker "NMT", Latitude 37° 02.7740540' N., Longitude 76° 32.0960864'
W.; thence to a VMRC Marker "NTH" located at Latitude 37° 03.2030055'
N., Longitude 76° 31.4231211' W.; thence to a point on the north shore of the
river at Blunt (Blount) Point, said point being in line with VMRC Markers
"NMT" and "NTH" and located at Latitude 37° 03.3805862' N.,
Longitude 76° 31.1444562' W.
"Unassigned ground" means all grounds not assigned
pursuant to §§ 28.2-600 through 28.2-633 of the Code of Virginia,
established pursuant to § 28.2-551 of the Code of Virginia, or set aside
by court order, or those grounds set aside by declarations or regulation by the
Marine Resources Commission, and may be redefined by any of these legal
authorities.
"Upper Chesapeake Bay - Blackberry Hangs Area"
means all public grounds and unassigned grounds, in that area of the Chesapeake
Bay, bounded by a line, beginning at a point approximately 300 feet east of the
mean low water line of the Chesapeake Bay and approximately 1,230 feet
southwest of the end of the southern-most stone jetty at the mouth of the
Little Wicomico River, said point being Corner 1, Latitude 37° 53.1811193' N.,
Longitude 76° 14.1740146' W.; thence east-southeasterly to Corner 2, Latitude
37° 52.9050025' N., Longitude 76° 11.9357257' W.; thence easterly to Corner 3,
Latitude 37° 52.9076552' N., Longitude 76° 11.6098145' W.; thence southwesterly
to Corner 4, Latitude 37° 52.8684955' N., Longitude 76° 11.6402444' W.; thence
east-southeasterly to Corner 5, Latitude 37° 52.7924853' N., Longitude 76°
11.0253352' W.; thence southwesterly to Corner 6, Latitude 37° 49.4327736' N.,
Longitude 76° 13.2409959' W.; thence northwesterly to Corner 7, Latitude 37°
50.0560555' N., Longitude 76° 15.0023234' W.; thence north-northeasterly to
Corner 8, Latitude 37° 50.5581183' N., Longitude 76° 14.8772805' W.; thence
north-northeasterly to Corner 9, Latitude 37° 52.0260950' N., Longitude 76°
14.5768550' W.; thence northeasterly to Corner 1, said corner being the point
of beginning.
"Yeocomico River Area" means that area of the North
West Yeocomico River, inside Public Ground 8 of Westmoreland County and those
areas of the South Yeocomico River inside Public Grounds 102, 104, 106, and 107
of Northumberland County.
Public Ground 8 of Westmoreland County is located in the North
West Yeocomico River, beginning at a point approximately 1,455 feet northeast
of Crow Bar and 1,850 feet northwest of White Point, said point being Corner 1,
located at Latitude 38° 02.7468214' N., Longitude 76° 33.0775726' W.; thence
southeasterly to Corner 2, Latitude 38° 02.7397202' N., Longitude 76° 33.0186286'
W.; thence southerly to Corner 3, Latitude 38° 02.6021644' N., Longitude 76°
33.0234175' W.; thence westerly to Corner 4, Latitude 38° 02.6006669' N.,
Longitude 76° 33.0824799' W.; thence northerly to Corner 1, said corner being
the point of beginning.
Public Ground 102 of Northumberland County is located in the
South Yeocomico River, beginning at a point approximately 630 feet south of
Mundy Point and 1,745 feet southwest of Tom Jones Point, said point being
Corner 1, located at Latitude 38° 01.2138059' N., Longitude 76° 32.5577201' W.;
thence east-northeasterly to Corner 2, Latitude 38° 01.2268644' N., Longitude
76° 32.4497849' W.; thence southwesterly to Corner 3, Latitude 38° 01.1091209'
N., Longitude 76° 32.5591101' W.; thence northerly to Corner 1, said corner
being the point of beginning.
Public Ground 104 of Northumberland County is located in the
South Yeocomico River, beginning at a point approximately 670 feet north of
Walker Point and 1,900 feet northwest of Palmer Point, said point being Corner
1, located at Latitude 38° 00.8841841' N., Longitude 76° 32.6106215' W.; thence
southeasterly to Corner 2, Latitude 38° 00.8609163' N., Longitude 76°
32.5296302' W.; thence southeasterly to Corner 3, Latitude 38° 00.6693092' N.,
Longitude 76° 32.4161866' W.; thence southwesterly to Corner 4, Latitude 38°
00.6418466' N., Longitude 76° 32.5394849' W.; thence northwesterly to Corner 1,
said corner being the point of beginning.
Public Ground 106 of Northumberland County is located in
Palmer Cove of the South Yeocomico River, beginning at a point, on the mean low
water line approximately 2,000 feet east of northern headland of Palmer Cove,
said point being Corner 1, located at Latitude 38° 00.6914018' N., Longitude
76° 31.8629027' W.; thence southwesterly to Corner 2, Latitude 38° 00.6685187'
N., Longitude 76° 31.8798151' W.; thence westerly to Corner 3, Latitude 38°
00.6614246' N., Longitude 76° 32.1278647' W.; thence northerly to Corner 4,
Latitude 38° 00.7079228' N., Longitude 76° 32.1338276' W., said point being a
point on the mean low water line; thence following the mean low water line in a
clockwise direction to Corner 1, said corner being the point of beginning.
Public Ground 107 of Northumberland County is located in the
South Yeocomico River, beginning at a point approximately 1,000 feet southwest
of Barn Point and 1,300 feet northwest of Tom Jones Point, said point being
Corner 1, located at Longitude 38° 01.1389367' N., Latitude 76° 32.3425617' W.;
thence east-southeasterly to Corner 2, Latitude 38° 01.4106421' N., Longitude
76° 32.1077962' W.; thence southwesterly to Corner 3, Latitude 38° 01.2717197'
N., Longitude 76° 32.2917989' W.; thence north-northwesterly to Corner 1, said
corner being the point of beginning.
"York River Rotation Area 1" means all public
grounds in the York River, within Gloucester County, between a line from Upper
York River Flashing Red Channel Marker "8", Latitude 37° 17.8863666'
N., Longitude 76° 34.6534166' W.; thence northeasterly to Red Day Marker
"2" at the mouth of Cedar Bush Creek, Latitude 37° 18.6422166' N.,
Longitude 76° 33.8216000' W.; upstream to a line from the Flashing Yellow VIMS
Data Buoy "CB", Latitude 37° 20.4670000' N., Longitude 76°
37.4830000' W.; thence northeasterly to the inshore end of the wharf at Clay
Bank.
"York River Rotation Area 2" means all public
grounds in the York River, within Gloucester County, from the George P. Coleman
Memorial Bridge (U.S. Route 17), upstream to a line from Upper York River
Flashing Red Channel Marker "8", Latitude 37° 17.8863666' N.,
Longitude 76° 34.6534166' W.; thence northeasterly to Red Day Marker
"2" at the mouth of Cedar Bush Creek, Latitude 37° 18.6422166' N.,
Longitude 76° 33.8216000' W.
4VAC20-720-40. Open oyster harvest season and areas.
A. It shall be unlawful for any person to harvest oysters
from public and unassigned grounds outside of the seasons and areas set forth
in this section.
B. It shall be unlawful to harvest clean cull oysters from
the public oyster grounds and unassigned grounds except during the lawful
seasons and from the lawful areas as described in the following subdivisions of
this subsection.
1. James River Seed Area, including the Deep Water Shoal State
Replenishment Seed Area: October 1, 2016 2017, through April 30, 2017
2018.
2. Milford Haven: December 1, 2016 2017, through
February 28, 2017 2018.
3. Rappahannock River Area 9: November 1, 2016 2017,
through December 31, 2016 2017.
4. Little Wicomico River: October 1, 2016 2017,
through December 31, 2016 2017.
5. Coan River Area: October 1, 2016 2017,
through December 31, 2016 2017.
6. Yeocomico River Area: October 1, 2016 2017,
through December 31, 2016 2017. Except for Public Ground 106
that will be open December 1, 2016, through December 31, 2016.
7. Nomini Creek Area: October 1, 2016 2017,
through December 31, 2016 2017.
8. Mobjack Bay Area: October 1, 2017, through October 31,
2017 (hand tong only) and February 1, 2018, through February 28, 2018 (hand
scrape only).
8. 9. York River Rotation Area 2 1:
January 1, 2017 2018, through January 31, 2017 2018.
9. Rappahannock River Rotation Area 4: October 1, 2016,
through October 31, 2016, and December 1, 2016, through December 31, 2016.
10. Rappahannock River Rotation Area 2 1: November
1, 2016 October 1, 2017, through December 31, 2016 November
30, 2017.
11. Rappahannock River Rotation Area 6: November 1,
2017, through November 30, 2017 (patent tong only) and December 1, 2017,
through January 31, 2018 (hand scrape only).
12. Rappahannock River Area 7: December 1, 2017, through
January 31, 2018.
11. 13. Great Wicomico River Rotation
Area 1: December 1, 2016 2017, through January 31, 2017
2018.
12. 14. Upper Chesapeake Bay - Blackberry Hangs
Area: December 1, 2016 2017, through January 31, 2017 2018.
13. 15. James River Area and the Thomas Rock
Area (James River) Hand Scrape Areas 1 and 3: November 1, 2016
December 1, 2017, through January 31, 2017 February 28, 2018.
16. James River Hand Scrape Area 2: October 1,
2017, through December 31, 2017.
14. 17. Pocomoke and Tangier Sounds Rotation
Area 2 1: December 1, 2016 2017, through February
28, 2017 2018.
15. 18. Deep Rock Area: December 1, 2016 2017,
through February 28, 2017 2018.
16. 19. Seaside of the Eastern Shore (for clean
cull oysters only): November 1, 2016 2017, through March 31, 2017
2018.
C. It shall be unlawful to harvest seed oysters from the
public oyster grounds or unassigned grounds, except during the lawful seasons.
The harvest of seed oysters from the lawful areas is described in the following
subdivisions of this subsection.
1. James River Seed Area: October 1, 2016 2017,
through May 31, 2017 2018.
2. Deep Water Shoal State Replenishment Seed Area: October 1, 2016
2017, through May 31, 2017 2018.
4VAC20-720-60. Day and time limit.
A. It shall be unlawful to take, catch, or possess oysters on
Saturday and Sunday from the public oyster grounds or unassigned grounds in the
waters of the Commonwealth of Virginia, for commercial purposes, except that
this provision shall not apply to any person harvesting no more than one bushel
per day by hand or ordinary tong for household use only during the season when
the public oyster grounds or unassigned grounds are legally open for harvest.
B. From October 1, 2016, through December 31, 2016, it shall
be unlawful to take, catch, or possess oysters on any Friday from the public
oyster grounds or unassigned grounds described in 4VAC20-720-40 B 9 through B
14.
C. B. It shall be unlawful for any person to
harvest or attempt to harvest oysters prior to sunrise or after 2 p.m. from the
areas described in 4VAC20-720-40 B 1 through B 15 18 and
4VAC20-720-40 C. In addition, it shall be unlawful for any boat with an oyster
dredge or hand scrape aboard to leave the dock until one hour before
sunrise or return to the dock after sunset, and it shall be unlawful for any
boat with a hand scrape aboard to leave the dock until one-half hour before
sunrise or return to the dock after sunset.
4VAC20-720-70. Gear restrictions.
A. It shall be unlawful for any person to harvest oysters in
the James River Seed Area, including the Deep Water Shoal State Replenishment
Seed Area, the Rappahannock River Area 9, Milford Haven, Little Wicomico River,
Coan River Area, Nomini Creek Area and Yeocomico River Area,
except by hand tong. It shall be unlawful for any person to have a hand scrape
on board a boat that is harvesting or attempting to harvest oysters from public
grounds by hand tong.
B. It shall be unlawful to harvest oysters by any gear
from the seaside of the Eastern Shore area except by any gear,
except by hand or hand tong. It shall be unlawful to harvest oysters
that are not submerged at mean low water by any gear other than by hand.
C. It shall be unlawful to harvest oysters in the
Rappahannock River Rotation Areas 2 and 4 Area 1, Rappahannock
River Area 7, James River Area Hand Scrape Areas, Thomas
Rock Area, Upper Chesapeake Bay - Blackberry Hangs Area, York River
Area, and Great Wicomico River Area Areas by any gear except by
hand scrape.
D. It shall be unlawful to harvest oysters in the
Rappahannock River Rotation Area 6 by any gear except an oyster patent tong
from November 1, 2017, through November 30, 2017. It shall be unlawful to
harvest oysters in the Rappahannock River Rotation Area 6 by any gear except
hand scrape from December 1, 2017, through January 31, 2018.
E. It shall be unlawful to harvest oysters in the Mobjack
Bay Area by any gear except by hand tong from October 1, 2017, through October
31, 2017. It shall be unlawful to harvest oysters in the Mobjack Bay Area by
any gear except by hand scrape from February 1, 2018, through February 28,
2018.
D. F. It shall be unlawful for any person to
have more than one hand scrape on board any boat that is harvesting oysters or
attempting to harvest oysters from public grounds. It shall be unlawful for any
person to have a hand tong on board a boat that is harvesting or attempting to
harvest oysters from public grounds by hand scrape.
E. G. It shall be unlawful to harvest oysters
from the Pocomoke and Tangier Sounds Rotation Area 2 1, except by
an oyster dredge.
F. H. It shall be unlawful to harvest oysters
from the Deep Rock Area, except by an oyster patent tong.
4VAC20-720-75. Gear license.
A. It shall be unlawful for any person to harvest shellfish
from the hand scrape areas in the Rappahannock River, James River, Upper
Chesapeake Bay, York River Area, Mobjack Bay, and Great Wicomico
River unless that person has first obtained a valid hand scrape license.
B. It shall be unlawful for any person to harvest shellfish
with an oyster dredge from the public oyster grounds in the Pocomoke and
Tangier Sounds Rotation Area 2 1, unless that person has first
obtained a valid oyster dredge license.
C. It shall be unlawful for any person to harvest shellfish
with a patent tong from the public oyster grounds in the Deep Rock Area or
Rappahannock River Rotation Area 6 when open to patent tong use, unless
that person has first obtained a valid oyster patent tong license.
D. It shall be unlawful for any person to harvest shellfish
with a hand tong from the public oyster grounds, as described in 4VAC20-720-70
A, unless that person has first obtained a valid hand tong license.
E. It shall be unlawful for any person to harvest shellfish
by hand from the public oyster grounds on the seaside of the Eastern Shore, as
described in 4VAC20-720-70 B, unless that person has first obtained a valid
oyster by hand license. It shall be unlawful for any person to harvest shellfish
from the public oyster grounds on the seaside of the Eastern Shore by hand
tong, as described in 4VAC20-720-70 B, unless that person has first obtained a
valid oyster hand tong license.
4VAC20-720-80. Quotas and harvest limits.
A. It shall be unlawful for any person who does not possess a
valid commercial fisherman's registration license and a valid gear license
required by harvest area, as described in 4VAC20-720-75, and has not paid the
current year's oyster resource user fee to harvest or possess any oysters for
commercial purposes. Any individual who possesses the valid licenses and has
paid the oyster resource user fee as described in this subsection shall be
limited to a maximum harvest of eight bushels per day. It shall be unlawful for
any vessel to exceed a daily vessel limit of 24 16 bushels clean
cull oysters harvested from the areas described in 4VAC20-720-40 B 8 through 15
18.
B. It shall be unlawful for any person who does not possess a
valid commercial fisherman's registration license and a valid gear license
required by harvest area, as described in 4VAC20-720-75, and has not paid the
current year's oyster resource user fee to harvest or possess any oysters for
commercial purposes. Any individual who possesses the valid licenses and has
paid the oyster resource user fee as described in this subsection shall be
limited to a maximum harvest of eight bushels per day. It shall be unlawful for
any vessel to exceed a daily vessel limit for clean cull oysters harvested from
the areas described in 4VAC20-720-40 B 2 through 7 and 16 19,
whereby that vessel limit shall equal the number of registered commercial
fisherman licensees on board the vessel who hold a valid gear license and who
have paid the oyster resource user fee multiplied by eight.
C. It shall be unlawful for any vessel to exceed a daily
vessel limit for clean cull oysters harvested from the areas described in
4VAC20-720-40 B 1, whereby that vessel limit shall equal the number of
registered commercial fisherman licensees on board the vessel who hold a valid
gear license and who have paid the oyster resource user fee multiplied by 12.
It shall be unlawful for any person who does not possess a valid commercial
fisherman's registration license and hold a valid gear license required by
harvest area, as described in 4VAC20-720-75, and has not paid the current
year's oyster resource user fee to harvest or possess any oysters for commercial
purposes. Any individual who possesses the valid licenses and has paid the
oyster resource user fee as described in this subsection shall be limited to a
maximum harvest of 12 bushels per day.
D. In the Pocomoke and Tangier Sounds Rotation Area 2 1,
no blue crab bycatch is allowed. It shall be unlawful to possess on board any
vessel more than 250 hard clams.
4VAC20-720-90. Harvest permit required for the James River Area
and Thomas Rock Area Hand Scrape Areas.
A permit is required for the James River Area and the
Thomas Rock Area Hand Scrape Areas 1, 2, and 3. It shall be unlawful
for any person to harvest, or attempt to harvest, oysters from the James River Area
and the Thomas Rock Area Hand Scrape Areas without first obtaining a
harvest permit from the Marine Resources Commission as required by § 28.2-518
of the Code of Virginia.
4VAC20-720-100. Seed oyster planting procedures.
A. The marine police officer at the point of seed harvest may
require that a marine police officer be present during the seed planting. When
this is required, it will be specified on the seed transfer permit. If a marine
police officer is required to be present at planting, the planter shall notify
the marine police officer in the area prior to planting. It shall be unlawful for
the permittee or planter to plant the oysters without a marine police officer
being present.
B. The planting of seed oysters shall consist of spreading
the oysters loosely on the bottom of the planting area. It shall be unlawful to
plant seed oysters in any manner except by spreading the oysters loosely on the
bottom.
C. Seed oysters shall be placed on a designated and marked
area of the private ground from which said oysters are not to be removed until
after May 31. It shall be unlawful to reharvest these seed oysters prior to
June 1 December 31 if placed prior to December 31. Any seed oysters
placed January 1 through May 31 are not to be removed until after May 31. It
shall be unlawful to remove these seed oysters within 30 days of being placed
on private ground.
VA.R. Doc. No. R18-5251; Filed August 30, 2017, 12:49 p.m.
TITLE 8. EDUCATION
STATE BOARD OF EDUCATION
Final Regulation
Title of Regulation: 8VAC20-740. Regulations
Governing Nutritional Guidelines for Competitive Foods Sold in the Public
Schools (adding 8VAC20-740-10 through 8VAC20-740-40).
Statutory Authority: § 22.1-207.4 of the Code of
Virginia.
Effective Date: October 18, 2017.
Agency Contact: Lynne A. Fellin, Associate Director,
Office of School Nutrition Programs, Department of Education, 101 North 14th
Street, P.O. Box 2120, Richmond, VA 23228, telephone (804) 225-2717, or email
lynne.fellin@doe.virginia.gov.
Summary:
The amendments (i) establish nutritional standards for
competitive foods available for sale to students on the school campus of any
public school and other public school food authorities, such as residential
child care institutions, during the school day; (ii) require all local school
boards to adopt the nutritional standards as part of existing wellness
policies; (iii) establish recordkeeping requirements; and (iv) require the
Department of Education to ensure compliance with the standards.
The amendments are based on the Institute of Medicine's
Recommended Standards for Competitive Foods in Schools, align the regulation
with the U.S. Department of Agriculture interim final rule governing
competitive foods in schools issued in June 2013, and incorporate the
fundraiser exemptions required by Chapter 568 of the 2015 Acts of Assembly.
Summary of Public Comments and Agency's Response: A
summary of comments made by the public and the agency's response may be
obtained from the promulgating agency or viewed at the office of the Registrar
of Regulations.
CHAPTER 740
REGULATIONS GOVERNING NUTRITIONAL GUIDELINES STANDARDS FOR COMPETITIVE
FOODS SOLD AVAILABLE FOR SALE IN THE PUBLIC SCHOOLS
8VAC20-740-10. Definitions.
[ The following words and terms when used in
this chapter shall have the following meanings unless the context clearly
indicates otherwise: ]
"A la carte item" means an individually priced
food item served by the local school nutrition department that may or may not
be part of the reimbursable meal under the federal Child Nutrition Programs.
"After school activities" means activities that
occur on the school grounds campus after regular school hours the
school day.
"Beverage" means a drinkable liquid.
"Calorie" means the amount of heat required to
change the temperature of one gram of water from 14.5 degrees Celsius to 15.5
degrees Celsius. Calorie is used synonymously with kilocalorie as a unit of
measure for energy obtained from food and beverages.
"Child nutrition programs" means school meal
programs funded and regulated by the U.S. Department of Agriculture (USDA) and
includes the National School Lunch Program (NSLP), School Breakfast Program
(SBP), Afterschool Snack Programs (ASP), Child and Adult Care Food Program
(CACFP), Summer Food Service Program (SFSP), and Special Milk Program (SMP).
"Combination foods" means products that contain
two or more components representing two or more of the recommended food groups:
fruit, vegetable, dairy, protein, or grains.
"Competitive food" means any all food,
excluding beverages, sold available for sale to students on the school grounds
campus during regular the school hours that is not part of the
reimbursable meals served through the National School Lunch Program (NSLP),
School Breakfast Program (SBP), or Afterschool Snack Program (ASP) day
other than meals reimbursed under programs authorized by the Richard B. Russell
National School Lunch Act (42 USC § 1751 et seq.) and the Child Nutrition
Act of 1966 (42 USC § 1771 et seq.).
"Competitive food" includes all foods sold
available for sale to students:
1. In school cafeterias as a la carte items not offered
as a component of the planned reimbursable menu.
2. In vending machines located on school grounds during
regular school hours the school campus during the school day.
3. As fundraisers held on school grounds during regular
school hours the school campus during the school day.
4. In school snack bars on school grounds during regular
school hours the school campus during the school day.
5. In school stores operated on school grounds during
regular school hours the school campus during the school day by the school,
a student association, or other school-sponsored organization.
6. At school activities such as special fundraisers,
achievement rewards, classroom parties, school celebrations, classroom snacks,
or school meetings held on school grounds during regular school hours
the school campus during the school day.
7. In culinary education programs where food prepared as
part of the educational curriculum is sold to students; however, this provision
does not apply if food is sold to adults only.
This term does not apply to food a student brings from
home for consumption at school or items available for sale to adults only in
areas not accessible to students (e.g., teachers lounges).
"Dietary Guidelines for Americans" means
guidelines jointly issued by the U.S. Department of Health and Human Services
and U.S. Department of Agriculture and revised every five years and that
provide authoritative advice based on current scientific evidence and medical
knowledge for people two years of age and older about how good dietary habits
can promote health and reduce risk for major chronic diseases.
"Food of minimal nutritional value" or
"FMNV" means foods and beverages that are restricted by the U.S. Department
of Agriculture (7 CFR 210.11(a)(2) and subsection (a) of Appendix B to 7 CFR
Part 210 Definition) unless specifically exempted by USDA. The federal FMNV
definition is limited to the following four specific categories of foods and
beverages:
1. Soda water (any carbonated or aerated beverages,
i.e., beverages that are labeled as "aerated" or that bubble and fizz
for several minutes after opening).
2. Water ices (any frozen, sweetened water such as
"…sicles" and flavored ice with the exception of products that
contain fruit, fruit juice, milk, milk ingredients, or egg ingredients other
than egg whites).
3. Chewing gum (regular and sugarless).
4. Certain candies (regular and sugarless),
including:
a. Hard candy (e.g., sour balls, candy sticks, lollipops,
starlight mints, after-dinner mints, sugar wafers, rock candy, cinnamon candy).
b. Jellies and gums (e.g., gum drops, jelly beans, and
jellied and fruit-flavored slices and shapes).
c. Marshmallow candies, fondant, such as candy corn and
soft mints, licorice, spun candy, and candy coated popcorn.
"Entree item" means an item that is either (i) a
combination food of meat or meat alternate and whole grain rich food; (ii) a
combination food of vegetable or fruit and meat or meat alternate; or (iii) a
meat or meat alternate alone with the exception of yogurt; low-fat or reduced
fat cheese; nuts, seeds, and nut or seed butters; and meat snacks (e.g., dried
beef jerky).
[ "Fundraiser" means a school-sponsored
activity where food or nonfood items are sold on the school campus during
regular school hours by the school-sponsored organization to raise money for a
school-related program or activity. One fundraiser is defined as one or more
fundraising activities by one or more school-sponsored organizations that last
one school day.
"Fundraising exemption" means an exception that
allows a school-sponsored organization to sell on the school campus during
regular school hours (i) food or beverages that do not meet the nutrition
standards established in this chapter and (ii) items that do not meet the U.S.
Department of Agriculture's Smart Snacks in Schools regulation. Fundraisers
that sell nonfood items or that sell foods or beverages that meet the nutrition
standards are not subject to this chapter or the U.S. Department of
Agriculture's Smart Snacks in Schools regulation. ]
"Kcal" means kilocalorie, commonly known as
calorie, which is a unit of measure in the United States for energy obtained
from food and beverages. A kilocalorie is equal to 1,000 calories.
"Obesity" means obesity in children and
adolescents referring to the age-specific and sex-specific body mass index
(BMI) that is equal to or greater than the 95th percentile of the BMI charts of
the Centers for Disease Control and Prevention (CDC).
"Regular school hours" means the same as the
standard school day, as defined in 8VAC20-131-5, a calendar day that averages
at least five and one-half instructional hours for students in grades 1 through
12, excluding breaks for meals and recess, and a minimum of three instructional
hours for students in kindergarten. Regular school hours does not include
school-related activities or events that occur either before or after the
standard school day, such as clubs, yearbook, band and choir practice, student
government, drama, childcare programs, interscholastic sporting events, school
plays, band concerts, or other school-related programs.
[ "Regular school hours" means the standard
school day, as defined in 8VAC20-131-5, except for the purpose of fundraiser
exemptions, breaks for meals and recess are included in the regular school
hours. ]
"School campus" means, for the purpose of
competitive food standards implementation, all areas of the property under the
jurisdiction of the school that are accessible to students during the school
day.
"School day" means, for the purpose of
competitive food standards implementation, the period from the midnight before
to 30 minutes after the end of the official school day.
"School food authority" or "SFA"
means, under the federal child nutrition laws, the entity that is legally
responsible for the operations and administration of the local school nutrition
programs (i.e., school division).
"School Health Advisory Board" or
"SHAB" means an entity formed according to the provisions of §
22.1-275.1 of the Code of Virginia that assists in the development of wellness
policies as required by § 204 of Public Law 108-265 (42 USC § 1751 et seq.) and
develops an annual report of activities that is required to be submitted to the
Department of Education as amended.
"Trans fat" means food items containing
vegetable shortening, margarine, or any partially hydrogenated vegetable oil
unless the label required on the food, pursuant to applicable federal and state
law, lists the trans fat content as less than 0.5 zero grams per
serving.
"Wellness policy" means a policy required for
public schools participating in a nutrition program authorized by the Richard
B. Russell National School Lunch Act (42 USC § 1751 et seq.) or the
Child Nutrition Act of 1966 (42 USC § 1771 et seq.) that meets
minimum standards designed to support school environments that promote student
wellness.
"Whole grains" means grains that are made with
enriched and whole grain meal or flour in accordance with the most recent
grains guidance from the U.S. Department of Agriculture Food and Nutrition
Service.
"Whole-grain rich" means products that contain
at least 50% whole grains and the remaining grains in the product must be
enriched.
8VAC20-740-20. Applicability.
A. This regulation chapter shall apply to all
public school divisions, public schools, and public school food authorities
(SFAs) in the Commonwealth of Virginia.
B. [ This ] regulation
[ chapter Unless otherwise provided in this chapter, its
provisions ] shall not apply to beverages.
C. This regulation chapter shall apply to the
nutritional content of food items , excluding beverages, sold available
for sale to students on the school grounds campus of any public school during
regular school hours, and other public SFAs such as residential child care
institutions, during the school day. It shall include apply to:
1. Foods sold available for sale to students in
vending machines.
2. Foods sold available for sale to students as a la
carte items in the school cafeteria.
3. Foods sold available for sale to students at
snack bars and stores operated by the school, a student association, or other
school-sponsored organization.
4. Foods sold available for sale to students at
school activities such as fundraisers.
5. Foods available for sale to students by culinary or
other educational programs.
D. This regulation chapter shall not apply to the
nutritional content of foods and beverages:
1. Provided through the National School Lunch, School
Breakfast, and Afterschool Snack programs, as regulated by 7 CFR Part 210 and 7
CFR Part 220 as meals reimbursed under programs authorized by the Richard
B. Russell National School Lunch Act (42 USC § 1751 et seq.) and the Child
Nutrition Act of 1966 (42 USC § 1771 et seq.).
2. Sold Available for sale at snack bars, concession
stands, or athletic events after regular school hours the school day.
3. Sold Available for sale either during
intermission or immediately before or after athletics events scheduled after
the school day.
4. Sold Available for sale for school-related
fundraising activities that take place off the school grounds campus
[ or for exempt fundraisers as outlined in 8VAC20-740-35 ].
5. Sold Available for sale during activities that
take place after regular school hours the school day, such as clubs,
yearbook, band and choir practice, student government, drama, sports practices,
interscholastic sporting events, school plays, and band concerts.
6. Available for sale to adults only in areas not
accessible to students.
E. The requirements of this chapter supplement 8VAC20-290
and 8VAC20-580, which remain in effect.
8VAC20-740-30. Nutrition standards.
Competitive foods sold to students shall support the
Dietary Guidelines for Americans by complying with the following nutritional
standards:
A. The nutrition standards apply to all foods available
for sale to students (i) outside the school meal programs; (ii) on the school
campus; and (iii) at any time during the school day. The nutrition standards
shall be consistent with the most recent Dietary Guidelines for Americans.
B. To be allowable, a competitive food must (i) meet all
of the competitive food nutrient standards and (ii) must either:
1. Be a grain product that contains 50% or more whole
grains by weight or have as the first ingredient a whole grain (i.e.,
whole-grain rich);
2. Have as the first ingredient one of the nongrain major
food groups: fruits, vegetables, dairy, or protein foods (e.g., meat, beans,
poultry, seafood, eggs, nuts, seeds, etc.); [ or ]
3. Be a combination food that contains 1/4 cup of fruit or
vegetable [ .; or
4. Contain 10% of the Daily Value of a nutrient of
public health concern based on the most recent Dietary Guidelines for Americans
(i.e., calcium, potassium, vitamin D, or dietary fiber) for the period through
June 30, 2016. Effective July 1, 2016, this criterion is obsolete and may not
be used to qualify as a competitive food. ]
If water is the first ingredient, the second ingredient
must be one of the food items listed in this subsection.
C. General exemptions:
1. Fresh, canned, and frozen fruits or vegetables with no
added ingredients except water or, in the case of fruit, packed in 100% juice,
extra light, or light syrup are exempt from the nutrient standards.
2. Canned vegetables that contain a small amount of sugar
for processing purposes are also exempt from the nutrient standards.
3. An entree item offered as part of the national school
lunch program under 7 CFR Part 210 or the school breakfast program under 7 CFR
Part 220 is exempt from all competitive food standards if it is offered as a
competitive food on the day of, or the school day after, it is offered in the
lunch or breakfast program. Exempt entree items offered as a competitive food
must be offered in the same or smaller portion sizes as in the lunch or
breakfast program.
Side dishes offered as part of the lunch or breakfast
program and served a la carte must meet the nutrition standards in this
section.
D. The accompaniments to a competitive food item must be
included in the nutrient profile as a part of the food item served in
determining if an item meets the nutrition standards for competitive food. The
contribution of the accompaniments may be based on the average serving size of
the accompaniment used per item.
E. Nutrient standards:
1. Standard 1: Calories.
a. Snack items and side dishes sold a la carte (i) shall be
no more than 200 calories or less per portion item as served or
as packaged, including the calorie content in any accompaniments, such as
butter, cream cheese, and salad dressing, and (ii) must meet all other nutrient
standards.
b. A la carte entree items shall not exceed calorie
limits on comparable National School Lunch Program (NSLP) entrees. A la carte
entree items shall not provide more calories or larger portion sizes than the
comparable NSLP entree items. In accordance with 8VAC20-290-10, a la carte
entree items for sale to students shall be limited to those entree items
recognized as being components of the school breakfast program or school lunch
program meal patterns. Entree items sold a la carte, unless the entree item
meets the exemption for NSLP/SBP entree items in subdivision C 3 of this
section, shall (i) contain no more than 350 calories, including the calorie
content of any accompaniments, per item as served or as packaged, and (ii) meet
all of the other nutrient standards in this section.
c. The calories contained in any accompaniments must be
included in the nutrient profile as a part of the item served.
2. Standard 2: Fat.
a. Snacks and food items shall meet the following
criteria for dietary fat per portion or as packaged:
(1) No more than 35% of total calories from fat.
(2) Less than 10% of total calories from saturated fats.
(3) Zero grams of trans fat.
b. Exceptions: Nuts and seeds (allowed as combination
products as long as other nutrient standards are met; the fat content will not
count against the total fat content of the product).
Total fat. Competitive foods shall contain no more than 35%
of total calories from fat per item as packaged or served. Exemptions to the
total fat standard are granted for:
(1) Reduced fat cheese and part-skim mozzarella cheese.
This exemption does not apply to combination foods.
(2) Nuts, seeds, and nut or seed butters. This exemption
does not apply to combination foods that contain nuts, seeds, or nut or seed
butters, such as peanut butter and crackers and trail mix.
(3) Products consisting of only dried fruit with nuts or
seeds with no added nutritive sweeteners or fat.
(4) Seafood with no added fat.
b. Saturated fat. Competitive foods shall have less than
10% of total calories from saturated fat per item as packaged or served.
Exemptions to the saturated fat standard are granted for:
(1) Reduced fat cheese and part-skim mozzarella cheese.
This exemption does not apply to combination foods.
(2) Nuts, seeds, and nut or seed butters. This exemption
does not apply to combination foods that contain nuts, seeds, or nut or seed
butters, such as peanut butter and crackers and trail mix.
(3) Products consisting of only dried fruit with nuts or
seeds with no added nutritive sweeteners or fat.
c. Trans fat. Competitive foods must have zero grams of
trans fat per item as packaged or served.
3. Standard 3: Sugar. a. Snacks and food items shall
provide no more than 35% of calories from total sugars per portion or as
packaged b. Exceptions Total sugar shall be no more than 35% of weight per
item as packaged or served. Exemptions to the sugar standard are provided for:
(1) 100% fruits and fruit juices in all forms without
added sugars.
(2) 100% vegetables and vegetable juices without added
sugars.
(3) Unflavored nonfat and low-fat (1.0%) milk and
yogurt.
(4) Flavored nonfat and low-fat (1.0%) milk with no more
than 22 grams of total sugars per 8-ounce serving.
(5) Flavored nonfat and low-fat yogurt with no more than
30 grams of total sugars per 8-ounce serving.
a. Dried whole fruits or vegetables.
b. Dried whole fruit or vegetable pieces.
c. Dehydrated fruits or vegetables with no added nutritive
sweeteners.
d. Dried fruits with nutritive sweeteners that are required
for processing or palatability purposes.
4. Standard 4: Sodium.
a. Snack items shall meet a sodium content limit of 200
milligrams or less per portion or as packaged.
b. A la carte entree items recognized as being
components of the school breakfast program or school lunch program meal
patterns that are not part of the planned reimbursable menu shall meet a sodium
content of 480 milligrams or less per portion. Portion sizes for a la carte
entree items shall not be larger than the comparable portion size for NSLP
entree items
a. Sodium content in snacks (i) shall be no more than
[ 230 200 ] mg per item as packaged or
served, including the sodium content in any accompaniments, such as butter,
cream cheese, and salad dressing; and (ii) must meet all other nutrient
standards. [ Effective July 1, 2016, the sodium standard shall
be no more than 200 mg per item as packaged or served, including the sodium
content in any accompaniments. ]
b. Entree items sold a la carte, unless the entre item
meets the exemption for NSLP/SBP entree items in subdivision C 3 of this
section (i) shall have no more than 480 mg of sodium per item as packaged or
served, including the sodium content in any accompaniments, such as butter,
cream cheese, and salad dressing; and (ii) must meet all other nutrient
standards in this section.
5. Standard 5: Foods of minimal nutritional value. In
accordance with 8VAC20-290-10 and 7 CFR Part 210, all foods of minimal
nutritional value (FMNV) as defined in 8VAC20-740-10 shall be prohibited from
being sold to students on school grounds during regular school hours.
[ 8VAC20-740-35. Exemption to the nutrition
standards for school-sponsored fundraisers.
A. Each public school shall be permitted to conduct, on
the school campus during regular school hours, no more than 30 school-sponsored
fundraisers per school year during which food or beverages that do not meet the
nutrition standards in this chapter or in the U.S. Department of Agriculture's
regulations may be sold to students. School divisions are not required to allow
exemptions to the nutrition standards in this chapter for school-sponsored
fundraisers and may implement more restrictive guidelines as part of the local
wellness policy requirements outlined in 8VAC20-740-40 A.
B. One fundraiser is defined as one or more fundraising
activities that last one school day. If multiple school-sponsored organizations
conduct fundraisers on the same day, the combined activities shall be counted
as one fundraiser. If a fundraising activity lasts more than one school day,
each subsequent day's activity shall be considered as one fundraiser and shall
count toward the 30 exempt fundraisers total per year.
C. Any fundraiser that sells food or beverages, whether
the items meet the nutrition standards or are exempt from the nutrition
standards in this chapter, may not be conducted during school meal service
times. Pursuant to the Regulations Governing School Lunch Sale of Food Items
(8VAC20-290) and the Regulations for the School Breakfast Program (8VAC20-580),
any food or beverage item cannot be sold in competition with the National
School Lunch Program and School Breakfast Program from 6 a.m. until after the
close of the last breakfast period and from the beginning of the first lunch
period to the end of the last lunch period. Pursuant to 8VAC20-290 and
8VAC20-580, the income from any food or beverage sold to students during these
times shall accrue to the nonprofit School Nutrition Program account.
D. An exemption is not required for nonfood fundraisers or
for fundraisers that sell food or beverage items that meet the nutrition
standards in this chapter. ]
8VAC20-740-40. Implementation and compliance.
A. Each local school board shall incorporate and adopt these
the nutrition guidelines as part of its existing local wellness policy
standards in this chapter as a compulsory component of the divisionwide local
wellness policy mandated by federal regulation for all local education agencies
that participate in the national school lunch program. In addition to
incorporating the nutrition standards for competitive foods, the local wellness
policy shall (i) establish and identify school division leadership with the
authority to enforce the local wellness policy throughout the school campus;
(ii) establish specific goals for nutrition promotion, nutrition education,
physical activities, and other school-based activities that promote wellness;
and (iii) establish policies that address marketing and advertising of only foods
that meet the nutrition standards for competitive foods, serve to promote
student health, prevent childhood obesity, and combat problems associated with
poor nutrition and physical inactivity.
B. Each local school board shall submit annually to the
Department of Education the School Health Advisory Board (SHAB) Progress Report
as required by § 22.1-275.1 of the Code of Virginia. This report shall
include a status report on the development and implementation of the local
wellness policy. This report shall be used by the Department of Education to
monitor compliance with this chapter. Local educational agencies and school
food authorities must retain the records used to document compliance with this
chapter; that is, the documentation used to assess the nutritional profile of
the food item and determine whether a food item is an allowable competitive
food (e.g., the nutrition labels, recipes, or product specifications).
1. Local educational agencies:
a. Shall be responsible for maintaining records documenting
compliance with the competitive food nutrition standards for food available for
sale in areas that are outside of the control of the school nutrition programs
operation.
b. Shall be responsible for ensuring any organization or
school activity designated as responsible for food service at the various
venues in the school (other than the school nutrition programs) maintains
records documenting compliance with the competitive food nutrition standards.
c. [ Shall be responsible for maintaining
records each school year documenting the number of exempt fundraisers conducted
at each school within the local education agency.
d. ] Shall designate an individual at the
division or school level to monitor and ensure compliance with this chapter in
all areas that are outside the control of the school nutrition programs
operation. This designee shall not be school nutrition personnel.
2. The school food authority shall be responsible for
maintaining records for foods served under the auspices of the nonprofit school
nutrition programs account.
3. The Department of Education shall ensure that the local
education agencies and school food authorities comply with these nutrition
standards [ and shall provide guidance to schools on alternative
school-sponsored fundraisers that do not involve the sale of foods or beverages
to students and guidance on how to determine if foods and beverages sold at
school-sponsored fundraisers meet these standards ]. Noncompliance
determined by the local education agency, school food authority, or Department
of Education shall require corrective action.
VA.R. Doc. No. R11-2611; Filed August 21, 2017, 11:09 a.m.
TITLE 8. EDUCATION
VIRGINIA POLYTECHNIC INSTITUTE AND STATE UNIVERSITY
Final Regulation
REGISTRAR'S NOTICE:
Virginia Polytechnic Institute and State University is claiming an exemption
from the Administrative Process Act in accordance with § 2.2-4002 A 6 of
the Code of Virginia, which exempts educational institutions operated by the
Commonwealth.
Title of Regulation: 8VAC105-11. Parking and Traffic (amending 8VAC105-11-10).
Statutory Authority: § 23.1-1301 of the Code of
Virginia.
Effective Date: September 18, 2017.
Agency Contact: Lori Buchanan, Business Services
Specialist, Office of the Vice President for Policy and Government, 319 Burruss
Hall, Blacksburg, VA 24061, telephone (540) 231-9512, or email lorib90@vt.edu.
Summary:
The amendment updates the university's parking regulation
to reflect revised parking procedures.
8VAC105-11-10. Definitions.
The following words and terms when used in this chapter shall
have the following meanings unless the context clearly indicates otherwise:
"Parking and Traffic Procedures" means the Parking
and Traffic Procedures, Virginia Tech Parking Services, effective July 1,
2014 August 1, 2017.
"Virginia Tech" means Virginia Polytechnic
Institute and State University.
"University owned or leased property" means any
property owned, leased, or controlled by Virginia Tech.
DOCUMENTS INCORPORATED BY REFERENCE (8VAC105-11)
Parking and Traffic Procedures, Virginia Tech Parking
Services (rev. 7/14)
Parking
and Traffic Operational Manual, Volume 25, 2017-2018 Academic Year,
Virginia Tech Division of Operations, Parking and Transportation (rev. 8/2017)
VA.R. Doc. No. R18-5219; Filed August 29, 2017, 2:28 p.m.
TITLE 9. ENVIRONMENT
DEPARTMENT OF ENVIRONMENTAL QUALITY
Fast-Track Regulation
Title of Regulation: 9VAC15-11. Public Participation
Guidelines (amending 9VAC15-11-50).
Statutory Authority: §§ 2.2-4007.02, 58.1-3661, and
62.1-195.1 of the Code of Virginia.
Public Hearing Information: No public hearings are
scheduled.
Public Comment Deadline: October 18, 2017.
Effective Date: November 2, 2017.
Agency Contact: Melissa Porterfield, Department of
Environmental Quality, 629 East Main Street, P.O. Box 1105, Richmond, VA 23218,
telephone (804) 698-4238, FAX (804) 698-4019, or email
melissa.porterfield@deq.virginia.gov.
Basis: Section 2.2-4007.02 of the Administrative Process
Act requires agencies to develop and adopt public participation guidelines to
solicit input from interested parties during the development of regulations.
The Department of Environmental Quality (DEQ) previously adopted regulations
concerning public participation guidelines.
Purpose: State law requires DEQ to adopt public
participation guidelines to solicit input during the development of
regulations. Chapter 795 of the 2012 Acts of Assembly revised
§ 2.2-4007.02 B of the Code of Virginia to allow interested parties the
right to be accompanied by or represented by counsel during the formulation of
a regulation. Participation by the public in the regulatory process is
essential to assist DEQ in the promulgation of regulations that will protect
the public health and safety.
Rationale for Using Fast-Track Rulemaking Process: The
proposed amendments are expected to be noncontroversial and appropriate for
using the fast-track rulemaking process. The amendments to this regulation make
the regulation consistent with the Code of Virginia and the model public
participation guidelines developed by the Department of Planning and Budget.
Substance: The Code of Virginia allows interested
parties the right to be accompanied by or represented by counsel during the
formulation of a regulation. This language has been added to the regulation.
Issues: This regulatory change will benefit the public
and the agency. The regulatory change amends the regulation to be consistent
with the Code of Virginia. The regulatory change does not place any additional
requirements on the public or the agency; therefore, there are no disadvantages
to the public or the agency.
Department of Planning and Budget's Economic Impact
Analysis:
Summary of the Proposed Amendments to Regulation. Pursuant to
Chapter 795 of the 2012 Acts of Assembly,1 the
Department of Environmental Quality (Department) proposes to specify in this
regulation that interested persons shall be afforded an opportunity to be
accompanied by and represented by counsel or other representative when
submitting data, views, and arguments, either orally or in writing, to the
agency.
Result of Analysis. The benefits likely exceed the costs for
all proposed changes.
Estimated Economic Impact. The current Public Participation
Guidelines state that: "In considering any nonemergency, nonexempt
regulatory action, the agency shall afford interested persons an opportunity to
submit data, views, and arguments, either orally or in writing, to the
agency." The department proposes to append "and (ii) be accompanied
by and represented by counsel or other representative."
Chapter 795 of the 2012 Acts of Assembly added to
§ 2.2-4007.02 of the Code of Virginia, "Public participation
guidelines," that interested persons also be afforded an opportunity to be
accompanied by and represented by counsel or other representative. Since the
Code of Virginia already specifies that interested persons shall be afforded an
opportunity to be accompanied by and represented by counsel or other
representative, the Department's proposal to add this language to the
regulation will not change the law in effect, but will be beneficial in that it
will inform interested parties who read this regulation but not the statute of
their legal rights concerning representation.
Businesses and Entities Affected. The proposed amendment
potentially affects all individuals who comment on pending regulatory changes.
Localities Particularly Affected. The proposed amendment does
not disproportionately affect particular localities.
Projected Impact on Employment. The proposed amendment does not
significantly affect employment.
Effects on the Use and Value of Private Property. The proposed
amendment does not affect the use and value of private property.
Real Estate Development Costs. The proposed amendment does not
affect real estate development costs.
Small Businesses:
Definition. Pursuant to § 2.2-4007.04 of the Code of Virginia,
small business is defined as "a business entity, including its affiliates,
that (i) is independently owned and operated and (ii) employs fewer than 500
full-time employees or has gross annual sales of less than $6 million."
Costs and Other Effects. The proposed amendment does not affect
costs for small businesses.
Alternative Method that Minimizes Adverse Impact. The proposed
amendment does not adversely affect small businesses.
Adverse Impacts:
Businesses. The proposed amendment does not adversely affect
businesses.
Localities. The proposed amendment does not adversely affect
localities.
Other Entities. The proposed amendment does not adversely
affect other entities.
________________________________
1 See http://leg1.state.va.us/cgi-bin/legp504.exe?121+ful+CHAP0795+hil
Agency's Response to Economic Impact Analysis: The
Department of Environmental Quality has no comment on the economic impact
analysis.
Summary:
Pursuant to § 2.2-4007.02 of the Code of
Virginia, the amendment provides that interested persons submitting data,
views, and arguments on a regulatory action may be accompanied by and
represented by counsel or another representative.
Part III
Public Participation Procedures
9VAC15-11-50. Public comment.
A. In considering any nonemergency, nonexempt regulatory
action, the agency shall afford interested persons an opportunity to (i)
submit data, views, and arguments, either orally or in writing, to the agency;
and (ii) be accompanied by and represented by counsel or other representative.
Such opportunity to comment shall include an online public comment forum on the
Town Hall.
1. To any requesting person, the agency shall provide copies
of the statement of basis, purpose, substance, and issues; the economic impact
analysis of the proposed or fast-track regulatory action; and the agency's
response to public comments received.
2. The agency may begin crafting a regulatory action prior to
or during any opportunities it provides to the public to submit comments.
B. The agency shall accept public comments in writing after
the publication of a regulatory action in the Virginia Register as follows:
1. For a minimum of 30 calendar days following the publication
of the notice of intended regulatory action (NOIRA).
2. For a minimum of 60 calendar days following the publication
of a proposed regulation.
3. For a minimum of 30 calendar days following the publication
of a reproposed regulation.
4. For a minimum of 30 calendar days following the publication
of a final adopted regulation.
5. For a minimum of 30 calendar days following the publication
of a fast-track regulation.
6. For a minimum of 21 calendar days following the publication
of a notice of periodic review.
7. Not later than 21 calendar days following the publication
of a petition for rulemaking.
C. The agency may determine if any of the comment periods listed
in subsection B of this section shall be extended.
D. If the Governor finds that one or more changes with
substantial impact have been made to a proposed regulation, he may require the
agency to provide an additional 30 calendar days to solicit additional public
comment on the changes in accordance with § 2.2-4013 C of the Code of Virginia.
E. The agency shall send a draft of the agency's summary
description of public comment to all public commenters on the proposed
regulation at least five days before final adoption of the regulation pursuant
to § 2.2-4012 E of the Code of Virginia.
VA.R. Doc. No. R18-5107; Filed August 25, 2017, 8:16 a.m.
TITLE 9. ENVIRONMENT
STATE WATER CONTROL BOARD
Fast-Track Regulation
Title of Regulation: 9VAC25-11. Public Participation
Guidelines (amending 9VAC25-11-50).
Statutory Authority: §§ 2.2-4007.02 and 62.1-44.15
of the Code of Virginia.
Public Hearing Information: No public hearings are
scheduled.
Public Comment Deadline: October 18, 2017.
Effective Date: November 2, 2017.
Agency Contact: Melissa Porterfield, Department of
Environmental Quality, 629 East Main Street, P.O. Box 1105, Richmond, VA 23218,
telephone (804) 698-4238, FAX (804) 698-4019, or email melissa.porterfield@deq.virginia.gov.
Basis: Section 2.2-4007.02 of the Administrative Process
Act requires agencies to develop and adopt public participation guidelines to
solicit input from interested parties during the development of regulations.
The State Water Control Board previously adopted regulations concerning public
participation guidelines using the regulatory development process.
Purpose: State law requires the State Water Control
Board to adopt public participation guidelines to solicit input during the
development of regulations. Chapter 795 of the 2012 Acts of Assembly revised
§ 2.2-4007.02 B of the Code of Virginia to allow interested parties the
right to be accompanied by or represented by counsel during the formulation of
a regulation. Participation by the public in the regulatory process is
essential to assist the State Water Control Board in the promulgation of
regulations that will protect the public health and safety.
Rationale for Using Fast-Track Rulemaking Process: The
proposed amendments are expected to be noncontroversial and appropriate for
using the fast-track rulemaking process. The amendments to this regulation make
the regulations consistent with the Code of Virginia and the model public
participation guidelines developed by the Department of Planning and Budget.
Substance: The Code of Virginia allows interested
parties the right to be accompanied by or represented by counsel during the
formulation of a regulation. This language has been added to the regulation.
Issues: This regulatory change will benefit the public
and the agency. The regulatory change amends the regulation to be consistent
with the Code of Virginia. The regulatory change does not place any additional
requirements on the public or the agency; therefore, there are no disadvantages
to the public or the agency.
Department of Planning and Budget's Economic Impact
Analysis:
Summary of the Proposed Amendments to Regulation. Pursuant to
Chapter 795 of the 2012 Acts of Assembly,1 the State Water Control
Board (Board) proposes to specify in this regulation that interested persons
shall be afforded an opportunity to be accompanied by and represented by
counsel or other representative when submitting data, views, and arguments,
either orally or in writing, to the agency.
Result of Analysis. The benefits likely exceed the costs for
all proposed changes.
Estimated Economic Impact. The current Public Participation
Guidelines state that: "In considering any nonemergency, nonexempt
regulatory action, the agency shall afford interested persons an opportunity to
submit data, views, and arguments, either orally or in writing, to the
agency." The Board proposes to append "and (ii) be accompanied by and
represented by counsel or other representative."
Chapter 795 of the 2012 Acts of Assembly added to § 2.2-4007.02
of the Code of Virginia "Public participation guidelines" that
interested persons also be afforded an opportunity to be accompanied by and
represented by counsel or other representative. Since the Code of Virginia
already specifies that interested persons shall be afforded an opportunity to
be accompanied by and represented by counsel or other representative, the
Board's proposal to add this language to the regulation will not change the law
in effect, but will be beneficial in that it will inform interested parties who
read this regulation but not the statute of their legal rights concerning
representation.
Businesses and Entities Affected. The proposed amendment
potentially affects all individuals who comment on pending regulatory changes.
Localities Particularly Affected. The proposed amendment does
not disproportionately affect particular localities.
Projected Impact on Employment. The proposed amendment does not
significantly affect employment.
Effects on the Use and Value of Private Property. The proposed
amendment does not affect the use and value of private property.
Real Estate Development Costs. The proposed amendment does not
affect real estate development costs.
Small Businesses:
Definition. Pursuant to § 2.2-4007.04 of the Code of Virginia,
small business is defined as "a business entity, including its affiliates,
that (i) is independently owned and operated and (ii) employs fewer than 500
full-time employees or has gross annual sales of less than $6 million."
Costs and Other Effects. The proposed amendment does not affect
costs for small businesses.
Alternative Method that Minimizes Adverse Impact. The proposed
amendment does not adversely affect small businesses.
Adverse Impacts:
Businesses. The proposed amendment does not adversely affect
businesses.
Localities. The proposed amendment does not adversely affect
localities.
Other Entities. The proposed amendment does not adversely
affect other entities.
________________________________
1 See http://leg1.state.va.us/cgi-bin/legp504.exe?121+ful+CHAP0795+hil
Agency's Response to Economic Impact Analysis: The State
Water Control board has no comment on the economic impact analysis.
Summary:
Pursuant to § 2.2-4007.02 of the Code of
Virginia, the amendment provides that interested persons submitting data,
views, and arguments on a regulatory action may be accompanied by and
represented by counsel or another representative.
Part III
Public Participation Procedures
9VAC25-11-50. Public comment.
A. In considering any nonemergency, nonexempt regulatory
action, the agency shall afford interested persons an opportunity to (i)
submit data, views, and arguments, either orally or in writing, to the agency;
and (ii) be accompanied by and represented by counsel or other representative.
Such opportunity to comment shall include an online public comment forum on the
Town Hall.
1. To any requesting person, the agency shall provide copies
of the statement of basis, purpose, substance, and issues; the economic impact
analysis of the proposed or fast-track regulatory action; and the agency's
response to public comments received.
2. The agency may begin crafting a regulatory action prior to
or during any opportunities it provides to the public to submit comments.
B. The agency shall accept public comments in writing after
the publication of a regulatory action in the Virginia Register as follows:
1. For a minimum of 30 calendar days following the publication
of the notice of intended regulatory action (NOIRA).
2. For a minimum of 60 calendar days following the publication
of a proposed regulation.
3. For a minimum of 30 calendar days following the publication
of a reproposed regulation.
4. For a minimum of 30 calendar days following the publication
of a final adopted regulation.
5. For a minimum of 30 calendar days following the publication
of a fast-track regulation.
6. For a minimum of 21 calendar days following the publication
of a notice of periodic review.
7. Not later than 21 calendar days following the publication
of a petition for rulemaking.
C. The agency may determine if any of the comment periods
listed in subsection B of this section shall be extended.
D. If the Governor finds that one or more changes with
substantial impact have been made to a proposed regulation, he may require the
agency to provide an additional 30 calendar days to solicit additional public
comment on the changes in accordance with § 2.2-4013 C of the Code of Virginia.
E. The agency shall send a draft of the agency's summary
description of public comment to all public commenters on the proposed
regulation at least five days before final adoption of the regulation pursuant
to § 2.2-4012 E of the Code of Virginia.
VA.R. Doc. No. R18-5105; Filed August 25, 2017, 8:20 a.m.
TITLE 9. ENVIRONMENT
STATE WATER CONTROL BOARD
Proposed Regulation
Title of Regulation: 9VAC25-260. Water Quality Standards (amending 9VAC25-260-140, 9VAC25-260-155, 9VAC25-260-170).
Statutory Authority: § 62.1-44.15 of the Code of Virginia; Clean Water Act (33 USC § 1251 et seq.); 40 CFR Part 131.
Public Hearing Information: No public hearings are scheduled.
Public Comment Deadline: November 17, 2017.
Agency Contact: David Whitehurst, Department of Environmental Quality, 629 East Main Street, P.O. Box 1105, Richmond, VA 23218, telephone (804) 698-4121, FAX (804) 698-4032, or email david.whitehurst@deq.virginia.gov.
Basis: Subdivision 3 a of § 62.1-44.15 of the Code of Virginia mandates and authorizes the State Water Control Board to (i) establish water quality standards and policies for any state waters consistent with the purpose and general policy of the State Water Control Law and (ii) modify, amend, or cancel any such standards or policies established. This provision requires the board to hold public hearings from time to time for the purpose of reviewing the water quality standards, and, as appropriate, adopting, modifying or canceling such standards.
The federal Clean Water Act (33 USC § 1251 et seq.) at § 303(c) mandates the State Water Control Board to review and, as appropriate, modify and adopt water quality standards. The corresponding federal water quality standards regulation at 40 CFR 131.6 describes the minimum requirements for water quality standards, which are use designations, water quality criteria to protect the designated uses, and an antidegradation policy. All of the citations mentioned describe mandates for water quality standards.
The State Water Control Law (Title 62.1 of the Code of Virginia – Waters of the State, Ports and Harbors) authorizes the protection and restoration of the quality of state waters, the safeguarding the clean waters from pollution, prevention, and reduction of pollution, and the promotion of water conservation.
The authority to adopt standards as provided by the provisions in the previously referenced citations is mandated, although the specific standards to be adopted or modified are discretionary to the Environmental Protection Agency (EPA) and the Commonwealth.
Purpose: The rulemaking is essential to the protection of health, safety, or welfare of the citizens of the Commonwealth because proper water quality standards protect water quality and living resources of Virginia's waters for consumption of fish and shellfish, recreational uses, and conservation in general.
These standards will be used in setting Virginia Pollutant Discharge Elimination System Permit limits and for evaluating the waters of the Commonwealth for inclusion in the Clean Water Act § 305(b) water quality characterization report and on the § 303(d) list of impaired waters. Waters not meeting standards will require development of a total maximum daily load (TMDL) under the Clean Water Act at § 303(e). The Water Quality Standards are the cornerstone for all these other programs. The goal is to provide the citizens of the Commonwealth with a technical regulation that is protective of water quality in surface waters, reflects recent scientific information, reflects agency procedures, and is reasonable and practical.
The environment will benefit because implementation of these amendments will result in better water quality in the Commonwealth for recreation, consumption of fish and shellfish, and protection of aquatic life.
Substance:
Table of Parameters (Toxics) - 9VAC25-260-140. An amendment to the cadmium criteria for the protection of freshwater and saltwater aquatic life is based on more recent EPA guidance issued in 2016. The proposed cadmium criteria reflect toxicity data for 75 new species and 49 new genera, which result in modest changes to criteria.
Amendments are proposed to update 94 human health criteria parameters. EPA issued revised recommendations for 94 chemical pollutants in June 2015. Updated recommendations for human health parameters reflect the latest scientific information and EPA policies, including updated exposure factors (body weight, drinking water consumption rates, fish consumption rate, relative source contribution), bioaccumulation factors, and toxicity factors (reference dose, cancer slope factor). Each of these 94 chemical pollutants has two criteria – one for waters designated as public water supplies and one for all other state waters – for a total of 188 criteria concentrations. Inclusion of new data by EPA results in varying changes to these criteria; 127 are decreased (become more stringent), 57 are increased (become less stringent), two are unchanged, and two are new additions to the regulation.
Ammonia Criteria - 9VAC25-260-155. Included is a proposal to amend the section to include new nationally recommended aquatic life criteria, issued by EPA 2013, for ammonia in freshwater. Like the current criteria, the proposed criteria are calculated as a function of temperature and pH and account for the presence or absence of trout and early life stages of fish. The recalculated ammonia criteria now incorporate toxicity data for freshwater mussels in the family Unionidae, which are the most sensitive organisms in the recalculation data base. The new criteria are about twice as stringent as the existing criteria primarily because more recent toxicity data show that mussels and snails (including endangered species) are very sensitive to ammonia and the current ammonia criteria do not provide sufficient protection for these species. Site specific options to calculate criteria omitting mussel toxicity data are proposed to be used in waters where a demonstration has been made that mussels are absent; however, consultation with U.S. Fish and Wildlife Service and the Virginia Department of Game and Inland Fisheries indicate freshwater mussels should be considered ubiquitous in Virginia and likely to be present in any perennial waterbody.
Bacteria Criteria - 9VAC25-260-170. In October 2012, EPA finalized its updated recommended national water quality criteria for bacteria designed for the protection of recreational uses (swimming). Amendments are proposed to incorporate those updates into the Virginia water quality standards and are intended to replace the current bacteria criteria for the protection of the primary contact recreation use. The revised EPA recommendations include a geometric mean (GM) value as well as a statistical threshold value (STV). The GM is a never-to-be-exceeded value; the STV should not be exceeded by more than 10% of the samples taken.
Issues: The primary advantage to the public is that the updated numerical toxics criteria are based on better scientific information to protect water quality and human health. The disadvantage is that criteria that become more stringent may result in increased costs to the regulated community. However, the goal is to set realistic, protective goals in water quality management and to maintain the most scientifically defensible criteria in the water quality standards regulation. EPA has also provided guidance that these criteria are "approvable" under the Clean Water Act.
The advantage to the agency or the Commonwealth that will result from the adoption of these amendments will be more accurate and scientifically defensible permit limits, assessments; and clean-up plans (TMDLs). These are discussed under the "Purpose" section where the goals of the proposal, the environmental benefits, and the problems the proposal is intended to solve are discussed.
The regulated community will find the amendments pertinent to its operations, particularly where the numerical criteria are more stringent since that may require additional capital or operating costs for control in its discharge.
There is no disadvantage to the agency or the Commonwealth that will result from the adoption of these amendments.
Department of Planning and Budget's Economic Impact Analysis:
Summary of the Proposed Amendments to Regulation. The State Water Control Board (Board) proposes to adopt the most recent water quality standards recommended by the United States Environmental Protection Agency (EPA) for ammonia and cadmium criteria for protection of aquatic life, 94 chemical pollutant criteria, and the bacteria criteria and assessment methodology for protection of human health.
Result of Analysis. The proposed regulation may introduce substantial costs (possibly over one-half billion dollars) on affected point sources and will likely benefit aquatic life and human health. The costs that potentially impacted dischargers might have to spend on treatment upgrades to meet more stringent criteria depend on individual permit requirements that are site-specific and variable. As a result, there is insufficient data to accurately compare the magnitude of the benefits versus the costs. Detailed analysis of the benefits and costs are in the next section.
Estimated Economic Impact. This regulation establishes water quality standards for surface waters of the Commonwealth. Criteria are based on the maximum acceptable amount of pollutants that directly affect aquatic life and/or human health and that can be discharged into receiving waters and not exceed criteria protective of designated uses. Federal and state mandates in the Clean Water Act at § 303(c), 40 CFR 131 and the Code of Virginia in § 62.1-44.15(3a) require that these water quality standards be evaluated every three years. In addition, § 303(a) of the Clean Water Act requires the EPA to develop and publish water quality criteria that reflect the latest scientific knowledge. EPA recommendations are purely based on protection of aquatic life and human health and do not reflect consideration of economic impacts or the technological feasibility of meeting pollutant concentrations in ambient water. These criteria are not rules, nor do they automatically become part of a state's water quality standards. States may adopt the criteria that the EPA publishes, modify the EPA's criteria to reflect site-specific conditions, or adopt different criteria based on other scientifically defensible methods. The EPA must approve any new water quality standards adopted by a state before they can be used for Clean Water Act purposes. Should a state fail to update its standards, the EPA may adopt and enforce water quality criteria on behalf of the state. In this action, the Board proposes to adopt the most recent water quality standards recommended by the EPA. Once adopted, these criteria become the basis of establishing permit limits and Total Maximum Daily Loads (TMDLs).
Freshwater Ammonia Criteria for Protection of Aquatic Life.
In 2013, the EPA updated its 1999 recommendations for ambient freshwater ammonia criteria to reflect the newly discovered sensitive nature of freshwater mussels and snails to ammonia toxicity. According to the EPA1 "Freshwater mussels are highly sensitive to ammonia toxicity and represent the most sensitive species in the dataset for the criteria recommendations. New science has demonstrated that freshwater snails are also sensitive to ammonia toxicity. Both mussels and snails are important to the environment because they serve as food sources for other organisms in the food web and provide vital services in improving and maintaining water quality. Specifically, mussels are filter feeders and can filter nutrients, toxics, and other pollutants out of the water, thereby helping to control the levels of these pollutants and reduce exposure to humans and other aquatic organisms. Snails feed on organic debris including algae, which helps to reduce the effects of eutrophication and keeps bottom substrates clean for other benthic organisms."
The allowable total ammonia nitrogen level depends on several factors (i.e., whether it is for acute or chronic levels, whether trout are absent or present, various combinations of pH and temperature levels, whether mussels and early life stages of fish are absent or present). Thus, the proposed regulation contains hundreds of ammonia criteria in tables for various combinations of the relevant factors. The proposed ammonia criteria are more stringent than the current limits by a factor of between 2.2 times and 5.9 times for all possible combinations of pH and temperature. However, the proposed criteria are about twice as stringent as the current criteria based on an assumed pH of 7 and temperature of 20 degrees Celsius. Criteria that are more stringent can result in more stringent effluent limits for Virginia Pollutant Discharge Elimination System (VPDES) permitted dischargers. Those sources with monitoring requirements in their permit may also be affected if their discharges have the potential to exceed the proposed ammonia criteria. According to DEQ, the estimated number of potentially affected facilities due to the proposed amendments to the ammonia criteria is 370 and includes those facilities with effluent limitations and those with monitoring requirements but no limits.
The primary and most widespread potential cost increase associated with all of the proposed amendments in this action would be from meeting more stringent ammonia limits for municipal dischargers to comply with the revised ammonia criteria. A permit holder may reduce the ammonia discharge through nitrification, which would convert ammonia into nitrate-nitrogen and then discharge nitrate into the water. If nitrate cannot be discharged into the water because of permit limits, then the facility may install a nitrification/denitrification system, convert nitrate-nitrogen from the first step into the harmless gas form of nitrogen, and discharge into the air instead of water.
The facilities most likely to be affected are those in the Chesapeake Bay watershed with design flows less than 0.1 million gallons/day (MGD) located east of Interstate 95 and those with design flows less than 0.5 MGD west of I-95. Permittees with discharges outside of the Bay watershed, particularly those facilities that are large in volume compared to the receiving stream, may also have similar potential financial impacts.
According to DEQ, there are approximately 220 discharge permits issued in the Chesapeake Bay watershed with either ammonia limits or ammonia monitoring requirements. Although ammonia limits or monitoring requirements are part of the permits, it may be assumed those facilities with ammonia limits east of Interstate 95 with a design flow equal to or greater than 0.1 MGD and those with ammonia limits west of I-95 with a design flow equal to or greater than 0.5 MGD either currently have ammonia control requirements or will be required to nitrify/denitrify to comply with the total nitrogen waste load allocations of the Water Quality Planning Management Regulation (9VAC25-720 et seq.) and the Chesapeake Bay Watershed General Permit Regulation for Total Nitrogen and Total Phosphorus Discharges and Nutrient Trading (9VAC25-820). DEQ believes that those facilities utilizing a nitrification/denitrification wastewater treatment process to meet total nitrogen concentration limits greatly reduce the ammonia concentrations in effluent to very low levels and consequently will most likely meet the more stringent ammonia criteria without additional effort.
There are approximately 20 facilities east of Interstate 95 with flows less than 0.1 MGD. It is anticipated that these facilities have the greatest likelihood to incur impacts due to more stringent ammonia criteria. Of these, 17 now have numeric ammonia limits and it is likely they have nitrification capability to meet current limits. However, an upgrade and/or operational procedure modification may be necessary to comply with newer, more stringent ammonia limits.
There are approximately 119 facilities west of I-95 with design flows less than 0.5 MGD. It is anticipated that these facilities have the greatest likelihood to incur impacts due to more stringent ammonia criteria. All but 2 have numeric ammonia limits now and it is likely that the facilities with numeric limits have nitrification capability to meet current limits; however, an upgrade and/or operational procedure modification may be necessary to comply with newer, more stringent ammonia limits. It is unknown how many of these would install a simple nitrification system or an advanced nitrification/denitrification system.
There are approximately 150 discharge permits issued outside of the Chesapeake Bay watershed with either ammonia limits or ammonia monitoring requirements. It is possible that those with only monitoring requirements will incur costs should more stringent effluent limits be necessary. All but 8 have numeric ammonia limits now, and it is likely these facilities have nitrification capability to meet current limits; however, an upgrade and/or operational procedure modification may be necessary to comply with newer, more stringent ammonia limits.
DEQ estimates that a simple nitrification system costs about $372,000 for a 0.10 MGD sewage treatment plant. The cost of an advanced treatment system capable of both nitrification and denitrification can range from $750,000 to $8,195,000 depending on the current level of treatment and volume of discharge. These costs are one-time capital expenditures and are unlikely to recur during the useful life of the equipment; however, operations and maintenance costs would be ongoing. Operations and maintenance costs for nitrification/denitrification could be $23,000/a year for a 0.10-MGD plant to $195,000/a year for a 0.60-MGD plant.
As an example, for a totally new 0.7 MGD plant, roughly 50% of the cost of the new oxidation ditch, and 100% of the submerged diffused outfall, etc., is attributed to the cost for ammonia removal. In this case, roughly 9% of the total cost can be attributed to ammonia removal or roughly $500,000 of the $5,655,000 construction bid price.
In another example, a facility design flow upgrade from 4.0 to 6.5 MGD, the cost attributable to ammonia removal, is more complicated because the oxidation ditch volume is set, with no expansion of the aerator volume, but there is a hydraulic increase of the overall facility. Roughly 30% of the aeration system, filter, and digester upgrade costs and 100% of the integrated fixed-film activated sludge costs are attributable to ammonia removal. This adds up to about $1,720,700 or approximately 13% of the overall bid price of $13,278,600. It is estimated the cost per gallon of ammonia removal in the examples given above for the new construction is $0.71/gallon and cost per gallon for the upgrade is $0.26/gallon.
The Virginia Association of Municipal Wastewater Agencies (VAMWA) has prepared an estimate of economic impact of the proposed ammonia criteria on its members and other sewage treatment facilities. Utilizing the capital and operating and maintenance costs estimated by the EPA for various design ranges, the VAMWA's study estimates that capital costs will reach $512.3 million and ongoing operating and maintenance costs will be $33.6 million per year for 490 affected facilities in 2014 dollars. These costs are expected to be distributed over a 10-year period as VPDES permits are reissued with compliance schedules. The study projects much higher relative costs for smaller facilities such as schools and public rest stops compared to larger facilities. The VAMWA estimate does not address upgrades and costs for commercial or industrial facilities with direct discharge permits, upgrades and costs for pretreatment that public treatment facilities may require of commercial and industrial facilities that discharge into public collection systems, and development and implementation costs of TMDLs for additional waters that may be listed for aquatic life impairment as a result of more stringent criteria.
A TMDL is a plan to improve the quality of an impaired water body. Development of TMDLs requires significant amounts of labor to collect data, to determine land uses, animal densities, crop densities, the number of septic systems, contributions from point and nonpoint sources, and construction of a simulation model. DEQ usually incurs the development costs, but some funding is provided from the federal government. Implementation of a TMDL may represent significant costs to pollution sources as well. For example, fencing may be required to prevent direct deposition into water from cattle, a buffer area may be needed to function as a filter for agricultural runoff, and failing septic systems may have to be fixed. In addition to these, the implementation involves public participation, and staff travel, which add to the overall costs. There are various cost share and incentive programs for TMDL implementation. The magnitude of TMDL costs varies from project to project and is pollutant specific. For example, the cost of a bacteria TMDL project costs range from $41,000 to $145,000.
According to DEQ, there is currently one outstanding aquatic life use impairment attributed to ammonia that has yet to be prioritized. There are no ammonia related TMDLs at this time. However given the more stringent values proposed by this regulation, that situation could change. DEQ does not know the potential impact of this change on development and implementation costs of TMDLs because a TMDL determination is site specific.
There appears to be general consensus that the proposed ammonia criteria may have a substantial economic impact particularly on smaller facilities. In addition, there appears to be a general agreement on the unit cost estimates provided above for various facility design sizes. However, there appears to be a difference of opinion on how many facilities will be able to meet the proposed criteria without having to build a new facility or upgrade. For example, the VAMWA study presumes that a substantial number of major Chesapeake Bay watershed facilities that currently nitrify will not be able to meet permit limits while DEQ believes that they will.
The EPA allows certain flexibilities in adopting water quality criteria. For example, states are allowed to adopt site-specific criteria to take into account absence or presence of sensitive species. After consultation with the Virginia Department of Game and Inland Fisheries, Virginia Department of Conservation and Recreation, and United States Fish and Wildlife Service, the Board concluded that it would assume the presence of freshwater mussels in any perennial freshwater stream in Virginia but does propose to allow point sources to demonstrate an absence of sensitive species on a site-by-site basis. Thus, some sources may be able to avoid compliance costs if they can demonstrate lack of sensitive species in their locations. However, such a demonstration would likely cost some money.
The Board also proposes to allow compliance schedules longer than 5 years under certain conditions for reissuance of existing permits. These flexibilities would help sources comply with the new criteria to some degree.
Freshwater & Saltwater Cadmium Criteria for Protection of Aquatic Life.
In 2016, the EPA updated its 2001 recommended cadmium aquatic life ambient water quality criteria in order to reflect the newest toxicity data for 75 new species and 49 new genera. The Board proposes to adopt the EPA's recommended standard for cadmium. There are four aquatic life criteria (i.e., acute and chronic limits for freshwater and saltwater). The proposed cadmium criteria are more stringent than the current limits by a factor between 1.1 times and 2.2 times. Criteria that are more stringent may mean additional treatment is needed to remove more cadmium before discharging effluent into surface waters. Those permitted treatment plants with monitoring requirements in their permit may also be affected if their discharges have the potential to exceed the proposed criteria.
According to DEQ, there are 24 active discharge permits with either numeric cadmium limits or monitoring requirements. Of these, 10 have effluent limits and 14 have monitoring requirements but no limits. Monitoring requirements without discharge limits typically result from a permit review using a "Reasonable Potential Analysis" that indicates the facility may have a particular parameter in its effluent, ergo the monitoring requirement. The monitoring data is used in subsequent permit reissuances to determine if discharge limits should be included. Given that the cadmium freshwater criteria are becoming more stringent it is assumed facilities with only monitoring requirements may be the most likely to be affected.
Furthermore, the most likely impact expected is for industrial dischargers. However, DEQ has no cost information on retrofits for these types of facilities, and each would be unique due to the type of industry, wastewater characteristics and treatment technology used. Thus, there are no available estimates for the potential costs at this time. As far as TMDL costs, there is one aquatic life use impairment near Lake Anna with cadmium listed as the impairment cause, but it has yet to be put on the priority list and as such an active TMDL has yet to be developed. A more stringent cadmium standard may add additional waters to the impaired waters list but DEQ does not know if that is the case at this time because such determinations are site specific. On the other hand, more stringent cadmium criteria based on latest scientific information will likely provide better protection for aquatic life.
Water Quality Criteria for Protection of Human Health.
In 2015, the EPA published water quality criteria for the protection of human health for 94 chemical pollutants. The revisions stemmed from the latest scientific information and the EPA policies, including updated body weight, drinking water consumption rate, fish consumption rate, bioaccumulation factors, health toxicity values, and relative source contributions. Each pollutant has two criteria (i.e., one for public water supply and one for all other waters) for a total of 188 individual criteria concentrations. 57 of these criteria would become less stringent, 127 would become more stringent, 2 would be unchanged, and 2 are new additions and do not have criteria in the current regulation.
Though 127 criteria that are more stringent have the potential to increase compliance costs, according to DEQ, the majority of the human heath criteria pollutants tend to be rather exotic compounds and discharger specific. Thus, the potential compliance cost to dischargers is unknown at this time. In addition, it is noted that many of the human health criteria toxins are not monitored routinely unless there is a known or suspected problem. DEQ does not believe there will be additional TMDL designations because of this change but that expectation is uncertain.
Due to anti-backsliding rules, existing permit limits cannot be made less stringent. Thus, 57 less stringent criteria are unlikely to have an effect on current permit limits. However, potential new sources discharging one of these pollutants will be subject to less stringent limits and may avoid installing treatment systems. Thus, new sources may realize some cost savings in potential treatment costs.
127 more stringent and 2 new human health criteria have the potential to help reduce many types of illnesses including cancer. However, some of these rather exotic pollutants may not be present in the Commonwealth's surface waters. If this is the case, no immediate significant impact is likely to be realized, but if any discharge containing these chemicals is discovered, health risks originating from the drinking water and fish consumption may be reduced and the source may have to incur some additional compliance costs.
In short, very few limits are based on human health criteria so no significant impact from the amendments is expected. However, given the large number of human health criteria amendments, it is difficult to determine with certainty at this time what the cost savings or expenses may be.
Bacteria Criteria for Protection of Human Health.
The Board proposes to revise the bacteria criteria and assessment methodology for protection of human health. E. coli and Enterococci concentrations are used as bacteria indicators for the presence of illness inducing pathogens in freshwater and saltwater respectively.
The aim of the proposed changes is to align Virginia's methodology and criteria with those recommended by EPA, which are expressed in terms of a statistical threshold value (replacing the single sample maximum) and a geometric mean. The current assessment methodology for the single sample maximum allows no more than 10% of the total samples to exceed the criteria over the assessment period that is typically a six-year monitoring database. The proposed statistical threshold value is a similar measure utilized by EPA. Under the proposed regulation, no more than 10% of the total samples may exceed the statistical threshold value using all monitoring data collected up to a 90-day period. Bacteria criteria are also expressed in terms of a geometric mean, which can only be calculated under the current water quality standards using at least 4 observations taken within a 30-day period. The geometric mean standard is a "never-to-be-exceeded" value. Its exceedance puts the water body on the impaired waters list. The intent of the amendment is to switch to a 90-day assessment period to enable the use of more monitoring data, which will maximize the number of monitoring stations that are assessed against both geometric mean and statistical threshold value criteria. The proposed amendment will adopt 2012 EPA recommended statistical threshold values for E. coli and Enterococci concentrations and are higher than the current values used for the single sample maximum. The geometric mean concentrations remain unchanged.
The rationale behind the amendment is the proposed bacteria criteria represent the most recent scientific basis for criteria designed to protect primary contact recreational uses. Also, the Federal BEACH Act of 2000 requires that, not later than 36 months after the date of publication by the EPA of new or revised water quality criteria for pathogens or pathogen indicators, each state having coastal recreation waters shall adopt and submit to the EPA new or revised water quality standards for the coastal recreation waters of the state for all pathogens and pathogen indicators to which the new or revised water quality criteria are applicable. In this case, the most recent EPA criteria were published in 2012.
One of the consequences resulting from these changes is that more waters may be assessed as impaired for the recreational use. Exceedances of the bacteria criteria are the leading cause of TMDL designations; about 80% of existing impairments are due to high bacteria concentrations. There are currently 441 bacteria impairments that are waiting for a development of a TMDL. It is not expected amendments to bacteria criteria will affect dischargers as end-of-pipe limits for bacteria are set at the criterion. However, the number of TMDLs that must be developed may increase.
Businesses and Entities Affected. The proposed amendments particularly affect municipal wastewater treatment facilities and industrial plants that discharge to surface waters of the Commonwealth.
The estimated number of potentially affected facilities due to proposed amendments to the ammonia criteria is 370 (approximately 220 discharge permits issued in the Chesapeake Bay watershed and 150 discharge permits issued outside of the Chesapeake Bay watershed).
According to DEQ, there are 24 active discharge permits with either numeric cadmium limits or monitoring requirements.
The number of potentially effected facilities due to the amended human health criteria and bacteria criteria is not known.
The proposed changes may also affect new and expanded point sources as well as nonpoint sources in the future.
Localities Particularly Affected. The proposed changes apply statewide. Localities with permits that may have to upgrade or install new equipment will be particularly effected.
Projected Impact on Employment. The net impact on employment is not known. A facility requiring an upgrade or monitoring under the proposed regulations will have to hire labor to accomplish those goals.
However, increased costs may also discourage expansion or the building of new plants reducing demand for labor.
Effects on the Use and Value of Private Property. Facilities likely to be affected the most are municipal wastewater treatment facilities. To the extent the proposed more stringent requirements introduce additional compliance costs on privately owned facilities, their asset values should decrease.
The proposed changes also have the potential to affect private property prices through improvements in environmental quality. However, such effects are usually contingent upon noticeable improvements. Since the magnitude of likely effects on environment is not known, no conclusive statements can be made about the effect on the value of private property.
Real Estate Development Costs. The proposed amendments do not directly affect real estate development costs.
Small Businesses:
Definition. Pursuant to § 2.2-4007.04 of the Code of Virginia, small business is defined as "a business entity, including its affiliates, that (i) is independently owned and operated and (ii) employs fewer than 500 full-time employees or has gross annual sales of less than $6 million."
Costs and Other Effects. Some of the industrial plants that discharge to surface waters of the Commonwealth will be associated with small businesses. The costs and other effects on them are the same as discussed above.
Alternative Method that Minimizes Adverse Impact. There are no clear alternative methods that would both comply with the Clean Water Act and cost less.
Adverse Impacts:
Businesses. The adverse impact on businesses is the additional compliance costs discussed above.
Localities. The adverse impact on localities is the additional compliance costs discussed above.
Other Entities. The proposed amendments will not adversely affect other entities.
___________________________________________
1 https://www.epa.gov/sites/production/files/2015-08/documents/flexibilities-for-states-applying-epa-s-ammonia-criteria-recommendations.pdf
Agency's Response to Economic Impact Analysis: The department has reviewed the economic impact analysis prepared by the Department of Planning and Budget and has no comment.
Summary:
The proposed amendments update the ammonia criteria for the protection of freshwater aquatic life as well as implementation issues and impacts to regulated dischargers, revise the bacteria criteria for human health protection in recreation waters, revise the cadmium criteria for the protection of freshwater and saltwater aquatic life, and update 94 human health criteria parameters.
9VAC25-260-140. Criteria for surface water.
A. Instream water quality conditions shall not be acutely1 or chronically2 toxic except as allowed in 9VAC25-260-20 B (mixing zones). The following are definitions of acute and chronic toxicity conditions:
"Acute toxicity" means an adverse effect that usually occurs shortly after exposure to a pollutant. Lethality to an organism is the usual measure of acute toxicity. Where death is not easily detected, immobilization is considered equivalent to death.
"Chronic toxicity" means an adverse effect that is irreversible or progressive or occurs because the rate of injury is greater than the rate of repair during prolonged exposure to a pollutant. This includes low level, long-term effects such as reduction in growth or reproduction.
B. The following table is a list of numerical water quality criteria for specific parameters.
Table of Parameters6, 7 |
PARAMETER
CAS Number | USE DESIGNATION |
AQUATIC LIFE | HUMAN HEALTH |
FRESHWATER | SALTWATER | Public Water Supply3 | All Other Surface Waters4 |
Acute1 | Chronic2 | Acute1 | Chronic2 |
Acenapthene (μg/l)
83329 | | | | | 670 70
| 990 90
|
Acrolein (μg/l)
107028 | 3.0 | 3.0 | | | 6.1 3
| 9.3 400
|
Acrylonitrile (μg/l)
107131 Known or suspected carcinogen; human health criteria at risklevel 10-5. | | | | | 0.51 0.61
| 2.5 70
|
Aldrin (μg/l)
309002 Known or suspected carcinogen;human health criteria at risk level 10-5. | 3.0 | | 1.3 | | 0.00049 0.0000077
| 0.00050 0.0000077
|
Ammonia (μg/l)
766‑41‑7 Chronic criterion is a 30-day average concentration not to beexceeded more than once every three years on the average.(see 9VAC25-260-155) | | | | | | |
Anthracene (μg/l)
120127 | | | | | 8,300 300
| 40,000 400
|
Antimony (μg/l)
7440360 | | | | | 5.6 | 640 |
Arsenic (μg/l)5
7440382 | 340 | 150 | 69 | 36 | 10 | |
Bacteria
(see 9VAC25-260-160 and 9VAC25-260-170) | | | | | | |
Barium (μg/l)
7440393 | | | | | 2,000 | |
Benzene (μg/l)
71432 Known or suspected carcinogen; human health criteria at risklevel 10-5 | | | | | 22 5.8
| 510 160
|
Benzidine (μg/l)
92875 Known or suspected carcinogen; human health criteria at risklevel 10-5 | | | | | 0.00086 0.0014
| 0.0020 0.11
|
Benzo (a) anthracene (μg/l)
56553 Known or suspected carcinogen; human health criteria at risklevel 10-5 | | | | | 0.038 0.012
| 0.18 0.013
|
Benzo (b) fluoranthene (μg/l)
205992 Known or suspected carcinogen; human health criteria at risklevel 10-5 | | | | | 0.038 0.012
| 0.18 0.013
|
Benzo (k) fluoranthene(μg/l)
207089 Known or suspected carcinogen;human health criteria at risk level 10-5 | | | | | 0.038 0.12
| 0.18 0.13
|
Benzo (a) pyrene (μg/l)
50328 Known or suspected carcinogen; human health criteria at risklevel 10-5 | | | | | 0.038 0.0012
| 0.18 0.0013
|
Bis2-Chloroethyl Ether (μg/l)
111444 Known or suspected carcinogen; human health criteria at risklevel 10-5 | | | | | 0.30 | 5.3 22
|
Bis (chloromethyl) Ether
542881 Known or suspected carcinogen; human health criteria atrisk level 10-5 | | | | | 0.0015 | 0.17 |
Bis2-Chloroisopropyl Ether (Bis (2-Chloro-1-methylethyl)Ether) (μg/l)
108601 | | | | | 1,400 200
| 65,000 4,000
|
Bis2-Ethylhexyl Phthalate (μg/l)
117817 Known or suspected carcinogen; human health criteria at risklevel 10-5. Synonym = Di-2-Ethylhexyl Phthalate. | | | | | 12 3.2
| 22 3.7
|
Bromoform (μg/l)
75252 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 43 70
| 1,400 1,200
|
Butyl benzyl phthalate (μg/l)
85687 | | | | | 1,500 0.10
| 1,900 0.10
|
Cadmium (μg/l)5
7440439 Freshwater values are a function of total hardness as calcium carbonate (CaCO3) mg/l and the WER. The minimum hardness allowed for use in the equation below shall be 25 and the maximum hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion (μg/l)
WER e {1.128[In(hardness)] – 3.828}] e (0.9789[ln(hardness)]-3.866) (CFa) Freshwater chronic criterion (μg/l)
WER [e {0.7852[In(hardness)] – 3.490}] CFc e (0.7977[ln(hardness)]-3.909) (CFc) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140 F e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa = 1.136672-[(ln hardness)(0.041838)] CFc = 1.101672-[(ln hardness)(0.041838)] | 3.9 1.8
CaCO3 = 100
| 1.1 0.72
CaCO3 = 100
| 40 33
X WER
| 8.8 7.9
X WER
| 5 | |
Carbon tetrachloride (μg/l)
56235 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 2.3 4.0
| 16 50
|
Carbaryl (μg/l)
63252 | 2.1 | 2.1 | 1.6 | | | |
Chlordane (μg/l)
57749 Known or suspected carcinogen; human health criteria at risk level 10-5. | 2.4 | 0.0043 | 0.09 | 0.0040 | 0.0080 0.0031
| 0.0081 0.0032
|
Chloride (μg/l)
16887006 Human health criterion to maintain acceptable taste and aesthetic quality and applies at the drinking water intake. Chloride criteria do not apply in Class II transition zones (see subsection C of this section). | 860,000 | 230,000 | | | 250,000 | |
Chlorine, Total Residual (μg/l)
7782505 In DGIF class i and ii trout waters (9VAC25-260-390 through 9VAC25-260-540) or waters with threatened or endangered species are subject to the halogen ban (9VAC25-260-110). | 19 See 9VAC25-260-110 | 11 See 9VAC25-260-110 | | | | |
Chlorine Produced Oxidant (μg/l)
7782505 | | | 13 | 7.5 | | |
Chlorobenzene (μg/l)
108907 | | | | | 130 100
| 1,600 800
|
Chlorodibromomethane (μg/l)
124481 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 4.0 8.0
| 130 210
|
Chloroform (μg/l)
67663 | | | | | 340 60
| 11,000 2,000
|
2-Chloronaphthalene (μg/l)
91587 | | | | | 1,000 800
| 1,600 1,000
|
2-Chlorophenol (μg/l)
95578 | | | | | 81 30
| 150 800
|
Chlorpyrifos (μg/l)
2921882 | 0.083 | 0.041 | 0.011 | 0.0056 | | |
Chromium III (μg/l)5
16065831 Freshwater values are a function of total hardness as calcium carbonate CaCO3 mg/l and the WER. The minimum hardness allowed for use in the equation below shall be 25 and the maximum hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion μg/l WER [e{0.8190[In(hardness)]+3.7256}] (CFa) Freshwater chronic criterion μg/l
WER [e{0.8190[In(hardness)]+0.6848}] (CFc) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140.F e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa= 0.316 CFc=0.860 | 570
(CaCO3 = 100) | 74
(CaCO3 = 100) | | | 100 (total Cr) | |
Chromium VI (μg/l)5
18540299 | 16 | 11 | 1,100 | 50 | | |
Chrysene (μg/l)
218019 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.038 1.2
| 0.018 1.3
|
Copper (μg/l)5
7440508 Freshwater values are a function of total hardness as calcium carbonate CaCO3 mg/l and the WER. The minimum hardness allowed for use in the equation below shall be 25 and the maximum hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion (μg/l) WER [e {0.9422[In(hardness)]-1.700}] (CFa) Freshwater chronic criterion (μg/l)
WER [e {0.8545[In(hardness)]-1.702}] (CFc) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140 F. e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa = 0.960 CFc = 0.960 Alternate copper criteria in freshwater: the freshwater criteria for copper can also becalculated using the EPA 2007 Biotic Ligand Model (See 9VAC25-260-140 G). Acute saltwater criterion is a 24-hour average not to be exceeded more than once every three years on the average. | 13
CaCO 3 = 100 | 9.0
CaCO3 = 100 | 9.3
X WER | 6.0
X WER | 1,300 | |
Cyanide, Free (μg/l)
57125 | 22 | 5.2 | 1.0 | 1.0 | 140 4
| 16,000 400
|
DDD (μg/l)
72548 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.0031 0.0012
| 0.0031 0.0012
|
DDE (μg/l)
72559 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.0022 0.00018
| 0.0022 0.00018
|
DDT (μg/l)
50293 Known or suspected carcinogen; human health criteria at risk level 10-5. Total concentration of DDT and metabolites shall not exceed aquatic life criteria. | 1.1 | 0.0010 | 0.13 | 0.0010 | 0.0022 0.00030
| 0.0022 0.00030
|
Demeton (μg/l)
8065483 | | 0.1 | | 0.1 | | |
Diazinon (μg/l)
333415 | 0.17 | 0.17 | 0.82 | 0.82 | | |
Dibenz (a, h) anthracene (μg/l)
53703 Known or suspected carcinogen; human health criteria at risk level10-5. | | | | | 0.038 0.0012
| 0.18 0.0013
|
1,2-Dichlorobenzene (μg/l) 95501 | | | | | 420 1,000
| 1,300 3,000
|
1,3-Dichlorobenzene (μg/l) 541731 | | | | | 320 7
| 960 10
|
1,4 Dichlorobenzene (μg/l)
106467 | | | | | 63 300
| 190 900
|
3,3 Dichlorobenzidine (μg/l)
91941 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.21 0.49
| 0.28 1.5
|
Dichlorobromomethane(μg/l)
75274 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 5.5 9.5
| 170 270
|
1,2 Dichloroethane (μg/l)
107062 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 3.8 99
| 370 6,500
|
1,1 Dichloroethylene (μg/l)
75354 | | | | | 330 300
| 7,100 20,000
|
1,2-trans-dichloroethylene (μg/l)
156605 | | | | | 140 100
| 10,000 4,000
|
2,4 Dichlorophenol (μg/l)
120832 | | | | | 77 10
| 290 60
|
2,4 Dichlorophenoxy acetic acid (Chlorophenoxy Herbicide)(2,4-D) (μg/l)
94757 | | | | | 100 1,300
| 12,000 |
1,2-Dichloropropane (μg/l)
78875 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 5.0 9.0
| 150 310
|
1,3-Dichloropropene (μg/l)
542756 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 3.4 2.7
| 210 120
|
Dieldrin (μg/l)
60571 Known or suspected carcinogen; human health criteria at risk level 10-5. | 0.24 | 0.056 | 0.71 | 0.0019 | 0.00052 0.000012
| 0.00054 0.000012
|
Diethyl Phthalate (μg/l)
84662 | | | | | 17,000 600
| 44,000 600
|
2,4 Dimethylphenol (μg/l)
105679 | | | | | 380 100
| 850 3,000
|
Dimethyl Phthalate (μg/l)
131113 | | | | | 270,000 2,000
| 1,100,000 2,000
|
Di-n-Butyl Phthalate (μg/l)
84742 | | | | | 2,000 20
| 4,500 30
|
2,4 Dinitrophenol (μg/l)
51285 | | | | | 69 10
| 5,300 300
|
Dinitrophenols (μg/l)
25550587 | | | | | 10 | 1,000 |
2-Methyl-4,6-Dinitrophenol (μg/l)
534521 | | | | | 13 2
| 280 30
|
2,4 Dinitrotoluene (μg/l)
121142 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 1.1 0.49
| 34 17
|
Dioxin 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (μg/l)
1746016 | | | | | 5.0 E-8 | 5.1 E-8 |
1,2-Diphenylhydrazine (μg/l)
122667 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.36 0.3
| 2.0 |
Dissolved Oxygen (μg/l)
(See 9VAC25-260-50) | | | | | | |
Alpha-Endosulfan (μg/l)
959988 Total concentration alpha and beta-endosulfan shall not exceed aquatic life criteria. | 0.22 | 0.056 | 0.034 | 0.0087 | 62 20
| 89 30
|
Beta-Endosulfan (μg/l)
33213659 Total concentration alpha and beta-endosulfan shall not exceed aquatic life criteria. | 0.22 | 0.056 | 0.034 | 0.0087 | 62 20
| 89 40
|
Endosulfan Sulfate (μg/l)
1031078 | | | | | 62 20
| 89 40
|
Endrin (μg/l)
72208 | 0.086 | 0.036 | 0.037 | 0.0023 | 0.059 0.03
| 0.060 0.03
|
Endrin Aldehyde (μg/l)
7421934 | | | | | 0.29 1
| 0.30 1
|
Ethylbenzene (μg/l)
100414 | | | | | 530 68
| 2,100 130
|
Fecal Coliform
(see 9VAC25-260-160) | | | | | | |
Fluoranthene (μg/l)
206440 | | | | | 130 20
| 140 20
|
Fluorene (μg/l)
86737 | | | | | 1,100 50
| 5,300 70
|
Foaming Agents (μg/l) Criterion measured as methylene blue active substances. Criterion to maintain acceptable taste, odor, or aesthetic quality of drinking water and applies at the drinking water intake. | | | | | 500 | |
Guthion (μg/l)
86500 | | 0.01 | | 0.01 | | |
Heptachlor (μg/l)
76448 Known or suspected carcinogen; human health criteria at risk level 10-5. | 0.52 | 0.0038 | 0.053 | 0.0036 | 0.00079 0.000059
| 0.00079 0.000059
|
Heptachlor Epoxide (μg/l)
1024573 Known or suspected carcinogen; human health criteria at risk level 10-5. | 0.52 | 0.0038 | 0.053 | 0.0036 | 0.00039 0.00032
| 0.00039 0.00032
|
Hexachlorobenzene (μg/l)
118741 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.0028 0.00079
| 0.0029 0.00079
|
Hexachlorobutadiene (μg/l)
87683 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 4.4 0.1
| 180 0.1
|
Hexachlorocyclohexane Alpha-BHC (μg/l)
319846 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.026 0.0036
| 0.049 0.0039
|
Hexachlorocyclohexane Beta-BHC (μg/l)
319857 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.091 0.080
| 0.17 0.14
|
Hexachlorocyclohexane (μg/l) (Lindane) Gamma-BHC
58899 Known or suspected carcinogen; human health criteria at risk level 10-5.
| 0.95 | | 0.16 | | 0.98 4.2
| 1.8 4.4
|
Hexachlorocyclohexane (HCH)-Technical (μg/l) 608731 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.066 | 0.1 |
Hexachlorocyclopentadiene (μg/l)
77474 | | | | | 40 4
| 1,100 4
|
Hexachloroethane (μg/l)
67721 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 14 1
| 33 1
|
Hydrogen sulfide (μg/l)
7783064 | | 2.0 | | 2.0 | | |
Indeno (1,2,3,-cd) pyrene(μg/l)
193395 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.038 0.012
| 0.18 0.013
|
Iron (μg/l)
7439896 Criterion to maintain acceptable taste, odor or aesthetic quality of drinking water and applies at the drinking water intake. | | | | | 300 | |
Isophorone (μg/l)
78591 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 350 340
| 9,600 18,000
|
Kepone (μg/l)
143500 | | zero | | zero | | |
Lead (μg/l)5
7439921 Freshwater values are a function of total hardness as calcium carbonate CaCO3 mg/l and the water effect ratio. The minimum hardness allowed for use in the equation below shall be 25 and the maximum hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion (μg/l)
WER [e {1.273[In(hardness)]-1.084}](CFa) Freshwater chronic criterion (μg/l)
WER [e {1.273[In(hardness)]-3.259}] (CFc) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140 F e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa = 1.46203-[(ln hardness)(0.145712)] CFc = 1.46203-[(ln hardness)(0.145712)] | 94
CaCO3 = 100 | 11
CaCO3 = 100 | 230 X WER | 8.8 X WER | 15 | |
Malathion (μg/l)
121755 | | 0.1 | | 0.1 | | |
Mercury (μg/l) 5
7439976 | 1.4 | 0.77 | 1.8 | 0.94 | | |
Methyl Bromide (μg/l)
74839 | | | | | 47 100
| 1,500 10,000
|
3-Methyl-4-Chlorophenol
59507 | | | | | 500 | 2,000 |
Methyl Mercury (Fish Tissue Criterion mg/kg) 8
22967926 | | | | | 0.30 | 0.30 |
Methylene Chloride (μg/l)
75092 Known or suspected carcinogen; human health criteria at risk level 10-5. Synonym = Dichloromethane | | | | | 46 20
| 5,900 1,000
|
Methoxychlor (μg/l)
72435 | | 0.03 | | 0.03 | 100 0.02
| 0.02 |
Mirex (μg/l)
2385855 | | zero | | zero | | |
Nickel (μg/l)5
744002 Freshwater values are a function of total hardness as calcium carbonate CaCO3 mg/l and the WER. The minimum hardness allowed for use in the equation below shall be 25 and the maximum hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion (μg/l)
WER [e {0.8460[In(hardness)] + 1.312}] (CFa) Freshwater chronic criterion (μg/l)
WER [e {0.8460[In(hardness)] - 0.8840}] (CFc) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140 F e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa = 0.998 CFc = 0.997 | 180
CaCO3 = 100 | 20
CaCO3 = 100 | 74 X WER | 8.2 X WER | 610 | 4,600 |
Nitrate as N (μg/l)
14797558 | | | | | 10,000 | |
Nitrobenzene (μg/l)
98953 | | | | | 17 10
| 690 600
|
N-Nitrosodimethylamine (μg/l)
62759 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.0069 | 30 |
N-Nitrosodiphenylamine (μg/l)
86306 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 33 | 60 |
N-Nitrosodi-n-propylamine (μg/l)
621647 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.050 | 5.1 |
Nonylphenol (μg/l)
84852153 | 28 | 6.6 | 7.0 | 1.7 | | |
Parathion (μg/l)
56382 | 0.065 | 0.013 | | | | |
PCB Total (μg/l)
1336363 Known or suspected carcinogen; human health criteria at risk level 10-5. | | 0.014 | | 0.030 | 0.00064 | 0.00064 |
Pentachlorobenzene (μg/l)
608935 | | | | | 0.1 | 0.1 |
Pentachlorophenol (μg/l)
87865 Known or suspected carcinogen; human health criteria risk level at 10-5. Freshwater acute criterion (μg/l)
e (1.005(pH)-4.869) Freshwater chronic criterion (μg/l)
e (1.005(pH)-5.134) | 8.7
pH = 7.0 | 6.7
pH = 7.0 | 13 | 7.9 | 2.7 0.3
| 30 0.4
|
pH See 9VAC25-260-50 | | | | | | |
Phenol (μg/l)
108952 | | | | | 10,000 4,000
| 860,000 300,000
|
Phosphorus Elemental (μg/l)
7723140 | | | | 0.10 | | |
Pyrene (μg/l)
129000 | | | | | 830 20
| 4,000 30 |
Radionuclides | | | | | | |
Gross Alpha Particle Activity (pCi/L) | | | | | 15 | |
Beta Particle & Photon Activity (mrem/yr) (formerly man-made radionuclides) | | | | | 4 | |
Combined Radium 226 and 228 (pCi/L) | | | | | 5 | |
Uranium (μg/L) | | | | | 30 | |
Selenium (μg/l)5
7782492 WER shall not be used for freshwater acute and chronic criteria. Freshwater criteria expressed as total recoverable. | 20 | 5.0 | 290 X WER | 71
X WER | 170 | 4,200 |
Silver (μg/l)5
7440224 Freshwater values are a function of total hardness as calcium carbonate (CaCO3) mg/l and the WER. The minimum hardness allowed for use in the equation below shall be 25 and the maximum hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion (μg/l)
WER [e {1.72[In(hardness)]-6.52}] (CFa) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140 F e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa = 0.85 | 3.4; CaCO3 = 100 | | 1.9 X WER | | | |
Sulfate (μg/l) Criterion to maintain acceptable taste, odor or aesthetic quality of drinking water and applies at the drinking water intake. | | | | | 250,000 | |
Temperature See 9VAC25-260-50 | | | | | | |
1,2,4,5-Tetrachlorobenzene 95943 | | | | | 0.03 | 0.03 |
1,1,2,2-Tetrachloroethane (μg/l)
79345 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 1.7 2.0
| 40 30
|
Tetrachloroethylene (μg/l)
127184 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 6.9 100
| 33 290
|
Thallium (μg/l)
7440280 | | | | | 0.24 | 0.47 |
Toluene (μg/l)
108883 | | | | | 510 57
| 6,000 520 |
Total Dissolved Solids (μg/l)
Criterion to maintain acceptable taste, odor or aesthetic quality of drinking water and applies at the drinking water intake. | | | | | 500,000 | |
Toxaphene (μg/l)
8001352 Known or suspected carcinogen; human health criteria at risk level 10-5. | 0.73 | 0.0002 | 0.21 | 0.0002 | 0.0028 0.0070
| 0.0028 0.0071
|
Tributyltin (μg/l)
60105 | 0.46 | 0.072 | 0.42 | 0.0074 | | |
1, 2, 4 Trichlorobenzene (μg/l)
120821 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 35 0.71
| 70 0.76
|
1,1,1-Trichloroethane
71556 | | | | | 10,000 | 200,000 |
1,1,2-Trichloroethane (μg/l)
79005 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 5.9 5.5
| 160 89
|
Trichloroethylene (μg/l)
79016 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 25 6.0
| 300 70
|
2, 4, 5 –Trichlorophenol
95954 | | | | | 300 | 600 |
2, 4, 6-Trichlorophenol (μg/l)
88062 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 14 15
| 24 28
|
2-(2, 4, 5-Trichlorophenoxy) propionic acid (Silvex) (μg/l)
93721 | | | | | 50 | |
Vinyl Chloride (μg/l)
75014 Known or suspected carcinogen; human health criteria at risk level 10-5. | | | | | 0.25 0.22
| 24 16
|
Zinc (μg/l)5
7440666 Freshwater values are a function of total hardness as calcium carbonate (CaCO3) mg/l and the WER. The minimum hardness allowed for use in the equation below shall be 25 and the maximum, hardness shall be 400 even when the actual ambient hardness is less than 25 or greater than 400. Freshwater acute criterion (μg/l)
WER [e {0.8473[In(hardness)]+0.884}](CFa) Freshwater chronic criterion (μg/l)
WER [e{0.8473[In(hardness)]+0.884}] (CFc) WER = Water Effect Ratio = 1 unless determined otherwise under 9VAC25-260-140 F e = natural antilogarithm ln = natural logarithm CF = conversion factor a (acute) or c (chronic) CFa = 0.978 CFc = 0.986 | 120 CaCO3 = 100 | 120 CaCO3 = 100 | 90
X WER | 81
X WER | 7,400 | 26,000 |
1One hour average concentration not to be exceeded more than once every 3 years on the average, unless otherwise noted. 2Four-day average concentration not to be exceeded more than once every 3 years on the average, unless otherwise noted. 3Criteria have been calculated to protect human health from toxic effects through drinking water and fish consumption, unless otherwise noted and apply in segments designated as PWS in 9VAC25-260-390 through 9VAC25-260-540. 4Criteria have been calculated to protect human health from toxic effects through fish consumption, unless otherwise noted and apply in all other surface waters not designated as PWS in 9VAC25-260-390 through 9VAC25-260-540. 5Acute and chronic saltwater and freshwater aquatic life criteria apply to the biologically available form of the metal and apply as a function of the pollutant's water effect ratio (WER) as defined in 9VAC25-260-140 F (WER X criterion). Metals measured as dissolved shall be considered to be biologically available, or, because local receiving water characteristics may otherwise affect the biological availability of the metal, the biologically available equivalent measurement of the metal can be further defined by determining a water effect ratio (WER) and multiplying the numerical value shown in 9VAC25-260-140 B by the WER. Refer to 9VAC25-260-140 F. Values displayed above in the table are examples and correspond to a WER of 1.0. Metals criteria have been adjusted to convert the total recoverable fraction to dissolved fraction using a conversion factor. Criteria that change with hardness have the conversion factor listed in the table above. 6The flows listed below are default design flows for calculating steady state wasteload allocations unless statistically valid methods are employed which demonstrate compliance with the duration and return frequency of the water quality criteria. Aquatic Life: | Acute criteria | 1Q10 | Chronic criteria | 7Q10 | Chronic criteria (ammonia) | 30Q10 | Human Health: | Noncarcinogens | 30Q5 | Carcinogens | Harmonic mean |
The following are defined for this section: "1Q10" means the lowest flow averaged over a period of 1 day which on a statistical basis can be expected to occur once every 10 climatic years. "7Q10" means the lowest flow averaged over a period of 7 consecutive days that can be statistically expected to occur once every 10 climatic years. "30Q5" means the lowest flow averaged over a period of 30 consecutive days that can be statistically expected to occur once every 5 climatic years. "30Q10" means the lowest flow averaged over a period of 30 consecutive days that can be statistically expected to occur once every 10 climatic years. "Averaged" means an arithmetic mean. "Climatic year" means a year beginning on April 1 and ending on March 31. 7The criteria listed in this table are two significant digits. For other criteria that are referenced to other sections of this regulation in this table, all numbers listed as criteria values are significant. 8The fish tissue criterion for methylmercury applies to a concentration of 0.30 mg/kg as wet weight in edible tissue for species of fish and shellfish resident in a waterbody that are commonly eaten in the area and have commercial, recreational, or subsistence value. |
C. Application of freshwater and saltwater numericalcriteria. The numerical water quality criteria listed in subsection B of thissection (excluding dissolved oxygen, pH, temperature) shall be appliedaccording to the following classes of waters (see 9VAC25-260-50) and boundarydesignations:
CLASS OF WATERS | NUMERICAL CRITERIA |
I and II (Estuarine Waters) | Saltwater criteria apply |
II (Transition Zone) | More stringent of either the freshwater or saltwater criteria apply |
II (Tidal Freshwater), III, IV, V, VI and VII | Freshwater criteria apply |
The following describes the boundary designations for Class II, (estuarine, transition zone and tidal freshwater waters) by river basin:
1. Rappahannock Basin. Tidal freshwater is from the fall line of the Rappahannock River to the upstream boundary of the transition zone including all tidal tributaries that enter the tidal freshwater Rappahannock River.
Transition zone upstream boundary – N38° 4' 56.59"/W76° 58' 47.93" (430 feet east of Hutchinson Swamp) to N38° 5' 23.33"/W76° 58' 24.39" (0.7 miles upstream of Peedee Creek).
Transition zone downstream boundary – N37° 58' 45.80"/W76° 55' 28.75" (1,000 feet downstream of Jenkins Landing) to N37° 59' 20.07/W76° 53' 45.09" (0.33 miles upstream of Mulberry Point). All tidal waters that enter the transition zone are themselves transition zone waters.
Estuarine waters are from the downstream boundary of the transition zone to the mouth of the Rappahannock River (Buoy 6), including all tidal tributaries that enter the estuarine waters of the Rappahannock River.
2. York Basin. Tidal freshwater is from the fall line of the Mattaponi River at N37° 47' 20.03"/W77° 6' 15.16" (800 feet upstream of the Route 360 bridge in Aylett) to the upstream boundary of the Mattaponi River transition zone, and from the fall line of the Pamunkey River at N37° 41' 22.64"/W77° 12' 50.83" (2,000 feet upstream of Totopotomy Creek) to the upstream boundary of the Pamunkey River transition zone, including all tidal tributaries that enter the tidal freshwaters of the Mattaponi and Pamunkey Rivers.
Mattaponi River transition zone upstream boundary – N37° 39' 29.65"/W76° 52' 53.29" (1,000 feet upstream of Mitchell Hill Creek) to N37° 39' 24.20"/W76° 52' 55.87" (across from Courthouse Landing).
Mattaponi River transition zone downstream boundary – N37° 32' 19.76"/W76° 47' 29.41" (old Lord Delaware Bridge, west side) to N37° 32' 13.25"/W76° 47' 10.30" (old Lord Delaware Bridge, east side).
Pamunkey River transition zone upstream boundary – N37° 32' 36.63"/W76° 58' 29.88" (Cohoke Marsh, 0.9 miles upstream of Turkey Creek) to N37° 32' 36.51"/W76° 58' 36.48" (0.75 miles upstream of creek at Cook Landing).
Pamunkey River transition zone downstream boundary – N37° 31' 57.90"/W76° 48' 38.22" (old Eltham Bridge, west side) to N37° 32' 6.25"/W76° 48' 18.82" (old Eltham Bridge, east side).
All tidal tributaries that enter the transition zones of the Mattaponi and Pamunkey Rivers are themselves in the transition zone.
Estuarine waters are from the downstream boundary of the transition zones of the Mattaponi and Pamunkey Rivers to the mouth of the York River (Tue Marsh Light) including all tidal tributaries that enter the estuarine waters of the York River.
3. James Basin. Tidal freshwater is from the fall line of the James River in the City of Richmond upstream of Mayo Bridge to the upstream boundary of the transition zone, including all tidal tributaries that enter the tidal freshwater James River.
James River transition zone upstream boundary – N37° 14' 28.25"/W76° 56' 44.47" (at Tettington) to N37° 13' 38.56"/W76° 56' 47.13" (0.3 miles downstream of Sloop Point).
Chickahominy River transition zone upstream boundary – N37° 25' 44.79"/W77° 1' 41.76" (Holly Landing).
Transition zone downstream boundary – N37° 12' 7.23"/W76° 37' 34.70" (near Carters Grove Home, 1.25 miles downstream of Grove Creek) to N37° 9' 17.23"/W76° 40' 13.45" (0.7 miles upstream of Hunnicutt Creek). All tidal waters that enter the transition zone are themselves transition zone waters.
Estuarine waters are from the downstream transition zone boundary to the mouth of the James River (Buoy 25) including all tidal tributaries that enter the estuarine waters of the James River.
4. Potomac Basin. Tidal freshwater includes all tidal tributaries that enter the Potomac River from its fall line at the Chain Bridge (N38° 55' 46.28"/W77° 6' 59.23") to the upstream transition zone boundary near Quantico, Virginia.
Transition zone includes all tidal tributaries that enter the Potomac River from N38° 31' 27.05"/W77° 17' 7.06" (midway between Shipping Point and Quantico Pier) to N38° 23' 22.78"/W77° 1' 45.50" (one mile southeast of Mathias Point).
Estuarine waters includes all tidal tributaries that enter the Potomac River from the downstream transition zone boundary to the mouth of the Potomac River (Buoy 44B).
5. Chesapeake Bay, Atlantic Ocean, and small coastal basins. Estuarine waters include the Atlantic Ocean tidal tributaries, and the Chesapeake Bay and its small coastal basins from the Virginia state line to the mouth of the bay (a line from Cape Henry drawn through Buoys 3 and 8 to Fishermans Island), and its tidal tributaries, excluding the Potomac tributaries and those tributaries listed in subdivisions 1 through 4 of this subsection.
6. Chowan River Basin. Tidal freshwater includes the Northwest River and its tidal tributaries from the Virginia-North Carolina state line to the free flowing portion, the Blackwater River and its tidal tributaries from the Virginia-North Carolina state line to the end of tidal waters at approximately state route 611 at river mile 20.90, the Nottoway River and its tidal tributaries from the Virginia-North Carolina state line to the end of tidal waters at approximately Route 674, and the North Landing River and its tidal tributaries from the Virginia-North Carolina state line to the Great Bridge Lock.
Transition zone includes Back Bay and its tributaries in the City of Virginia Beach to the Virginia-North Carolina state line.
D. Site-specific modifications to numerical water quality criteria.
1. The board may consider site-specific modifications to numerical water quality criteria in subsection B of this section where the applicant or permittee demonstrates that the alternate numerical water quality criteria are sufficient to protect all designated uses (see 9VAC25-260-10) of that particular surface water segment or body.
2. Any demonstration for site-specific human health criteria shall be restricted to a reevaluation of the bioconcentration or bioaccumulation properties of the pollutant. The exceptions to this restriction are for site-specific criteria for taste, odor, and aesthetic compounds noted by double asterisks in subsection B of this section and nitrates.
3. Procedures for promulgation and review of site-specific modifications to numerical water quality criteria resulting from subdivisions 1 and 2 of this subsection.
a. Proposals describing the details of the site-specific study shall be submitted to the board's staff for approval prior to commencing the study.
b. Any site-specific modification shall be promulgated as a regulation in accordance with the Administrative Process Act (§ 2.2-4000 et seq. of the Code of Virginia). All site-specific modifications shall be listed in 9VAC25-260-310 (Special standards and requirements).
E. Variances to water quality standards.
1. A variance from numeric criteria may be granted to a discharger if it can be demonstrated that one or more of the conditions in 9VAC25-260-10 H limit the attainment of one or more specific designated uses.
a. Variances shall apply only to the discharger to whom they are granted and shall be reevaluated and either continued, modified or revoked at the time of permit issuance. At that time the permittee shall make a showing that the conditions for granting the variance still apply.
b. Variances shall be described in the public notice published for the permit. The decision to approve a variance shall be subject to the public participation requirements of the Virginia Pollutant Discharge Elimination System (VPDES) Permit Regulation, 9VAC25-31 (Permit Regulation).
c. Variances shall not prevent the maintenance and protection of existing uses or exempt the discharger or regulated activity from compliance with other appropriate technology or water quality-based limits or best management practices.
d. Variances granted under this section shall not apply to new discharges.
e. Variances shall be submitted by the department's Division of Scientific Research or its successors to the U.S. Environmental Protection Agency for review and approval or disapproval.
f. A list of variances granted shall be maintained by the department's Division of Scientific Research or its successors.
2. None of the variances in this subsection shall apply to the halogen ban section (9VAC25-260-110) or temperature criteria in 9VAC25-260-50 if superseded by § 316(a) of the Clean Water Act requirements. No variances in this subsection shall apply to the criteria that are designed to protect human health from carcinogenic and noncarcinogenic toxic effects (subsection B of this section) with the exception of the metals, and the taste, odor, and aesthetic compounds noted by double asterisks and nitrates, listed in subsection B of this section.
F. Water effect ratio.
1. A water effects ratio (WER) shall be determined by measuring the effect of receiving water (as it is or will be affected by any discharges) on the bioavailability or toxicity of a metal by using standard test organisms and a metal to conduct toxicity tests simultaneously in receiving water and laboratory water. The ratio of toxicities of the metal(s) in the two waters is the WER (toxicity in receiving water divided by toxicity in laboratory water equals WER). Once an acceptable WER for a metal is established, the numerical value for the metal in subsection B of this section is multiplied by the WER to produce an instream concentration that will protect designated uses. This instream concentration shall be utilized in permitting decisions.
2. The WER shall be assigned a value of 1.0 unless the applicant or permittee demonstrates to the department's satisfaction in a permit proceeding that another value is appropriate, or unless available data allow the department to compute a WER for the receiving waters. The applicant or permittee is responsible for proposing and conducting the study to develop a WER. The study may require multiple testing over several seasons. The applicant or permittee shall obtain the department's Division of Scientific Research or its successor approval of the study protocol and the final WER.
3. The Permit Regulation at 9VAC25-31-230 C requires that permit limits for metals be expressed as total recoverable measurements. To that end, the study used to establish the WER may be based on total recoverable measurements of the metals.
4. The WER is established in a permit proceeding, shall be described in the public notice associated with the permit proceeding, and applies only to the applicant or permittee in that proceeding. The department's action to approve or disapprove a WER is a case decision, not an amendment to the present regulation.
The decision to approve or disapprove a WER shall be subject to the public participation requirements of the Permit Regulation, Part IV (9VAC25-31-260 et seq.). A list of final WERs will be maintained by the department's Division of Scientific Research or its successor.
5. A WER shall not be used for the freshwater and saltwater chronic mercury criteria or the freshwater acute and chronic selenium criteria.
G. Biotic Ligand Model for copper. On a case-by-case basis, EPA's 2007 copper criteria (EPA-822-F-07-001) biotic ligand model (BLM) for copper may be used to determine alternate copper criteria for freshwater sites. The BLM is a bioavailability model that uses receiving water characteristics to develop site-specific criteria. Site-specific data for 10 parameters are needed to use the BLM. These parameters are temperature, pH, dissolved organic carbon, calcium, magnesium, sodium, potassium, sulfate, chloride, and alkalinity. If sufficient data for these parameters are available, the BLM can be used to calculate alternate criteria values for the copper criteria. The BLM would be used instead of the hardness-based criteria and takes the place of the hardness adjustment and the WER. A WER will not be applicable with the BLM.
9VAC25-260-155. Ammonia surface water quality criteria.
A. The Department of Environmental Quality, after consultation with the Virginia Department of Game and Inland Fisheries and the U.S. Fish and Wildlife Service, has determined that the majority of Virginia freshwaters are likely to contain, or have contained in the past, freshwater mussel species in the family Unionidae and contain early life stages of fish during most times of the year. Therefore, the ammonia criteria presented in subsections B and C of this section are designed to provide protection to these species and life stages. In an instance where it can be adequately demonstrated that either freshwater mussels or early life stages of fish are not present in a specific waterbody, potential options for alternate, site-specific criteria are presented in subsection D of this section. Acute criteria are a one-hour average concentration not to be exceeded more than once every three years1 on the average, and chronic criteria are 30-day average concentrations not to be exceeded more than once every three years on the average.2
B. The one-hour average concentration of total ammonia nitrogen (in mg N/L) in freshwater shall not exceed, more than once every three years on the average1, the acute criteria for total ammonia (in mg N/L) for freshwaters with trout absent or present are below:
Acute Ammonia Freshwater Criteria
Total Ammonia Nitrogen (mg N/L)
|
pH
| Trout Present
| Trout Absent
|
6.5
| 32.6
| 48.8
|
6.6
| 31.3
| 46.8
|
6.7
| 29.8
| 44.6
|
6.8
| 28.1
| 42.0
|
6.9
| 26.2
| 39.1
|
7.0
| 24.1
| 36.1
|
7.1
| 22.0
| 32.8
|
7.2
| 19.7
| 29.5
|
7.3
| 17.5
| 26.2
|
7.4
| 15.4
| 23.0
|
7.5
| 13.3
| 19.9
|
7.6
| 11.4
| 17.0
|
7.7
| 9.65
| 14.4
|
7.8
| 8.11
| 12.1
|
7.9
| 6.77
| 10.1
|
8.0
| 5.62
| 8.40
|
8.1
| 4.64
| 6.95
|
8.2
| 3.83
| 5.72
|
8.3
| 3.15
| 4.71
|
8.4
| 2.59
| 3.88
|
8.5
| 2.14
| 3.20
|
8.6
| 1.77
| 2.65
|
8.7
| 1.47
| 2.20
|
8.8
| 1.23
| 1.84
|
8.9
| 1.04
| 1.56
|
9.0
| 0.885
| 1.32
|


The acute criteria for trout present shall apply to all Class V-Stockable Trout Waters and Class VI-Natural Trout Waters as listed in 9VAC25-260-390 through 9VAC25-260-540. The acute criteria for trout absent apply to all other fresh waters.
To calculate total ammonia nitrogen acute criteria values in freshwater at different pH values than those listed in this subsection, use the following formulas equations and round the result to two significant digits:
Where trout are present absent:
Acute Criterion Concentration (mg N/L) =
| 0.275
| +
| 39.0
|
| (1 + 107.204-pH)
| (1 + 10pH-7.204)
|
| 0.7249 X ( | 0.0114 | + | 1.6181 | ) X MIN |
| 1 + 107.204-pH | 1 + 10pH-7.204 |
Where MIN = 51.93 or 23.12 X 100.036 X (20 – T), whichever is less
T = Temperature in oC
Or where trout are absent present, whichever of the below calculation results is less:
Acute Criterion Concentration (mg N/L) =
| 0.411
| +
| 58.4
|
| (1 + 107.204-pH)
| (1 + 10pH-7.204)
|
1The default design flow for calculating steady state wasteload allocations for the acute ammonia criterion is the 1Q10 (see 9VAC25-260-140 B footnote 10) unless statistically valid methods are employed which demonstrate compliance with the duration and return frequency of the water quality criteria.
| ( | 0.275 | + | 39.0 | ) |
| 1 + 107.204-pH | 1 + 10pH-7.204 |
or
| 0.7249 X ( | 0.0114 | + | 1.6181 | ) X (23.12 X 100.036X(20 – T)) |
| 1 + 107.204-pH | 1 + 10pH-7.204 |
T = Temperature in oC
B. C. The 30-day average concentration of chronic criteria for total ammonia nitrogen (in mg N/L) where freshwater mussels and early life stages of fish are present in freshwater shall not exceed, more than once every three years on the average2, the chronic criteria are below:
Chronic Ammonia Freshwater Criteria
Early Life Stages of Fish Present
Total Ammonia Nitrogen (mg N/L)
|
| Temperature (°C)
|
pH
| 0
| 14
| 16
| 18
| 20
| 22
| 24
| 26
| 28
| 30
|
6.5
| 6.67
| 6.67
| 6.06
| 5.33
| 4.68
| 4.12
| 3.62
| 3.18
| 2.80
| 2.46
|
6.6
| 6.57
| 6.57
| 5.97
| 5.25
| 4.61
| 4.05
| 3.56
| 3.13
| 2.75
| 2.42
|
6.7
| 6.44
| 6.44
| 5.86
| 5.15
| 4.52
| 3.98
| 3.50
| 3.07
| 2.70
| 2.37
|
6.8
| 6.29
| 6.29
| 5.72
| 5.03
| 4.42
| 3.89
| 3.42
| 3.00
| 2.64
| 2.32
|
6.9
| 6.12
| 6.12
| 5.56
| 4.89
| 4.30
| 3.78
| 3.32
| 2.92
| 2.57
| 2.25
|
7.0
| 5.91
| 5.91
| 5.37
| 4.72
| 4.15
| 3.65
| 3.21
| 2.82
| 2.48
| 2.18
|
7.1
| 5.67
| 5.67
| 5.15
| 4.53
| 3.98
| 3.50
| 3.08
| 2.70
| 2.38
| 2.09
|
7.2
| 5.39
| 5.39
| 4.90
| 4.31
| 3.78
| 3.33
| 2.92
| 2.57
| 2.26
| 1.99
|
7.3
| 5.08
| 5.08
| 4.61
| 4.06
| 3.57
| 3.13
| 2.76
| 2.42
| 2.13
| 1.87
|
7.4
| 4.73
| 4.73
| 4.30
| 3.78
| 3.32
| 2.92
| 2.57
| 2.26
| 1.98
| 1.74
|
7.5
| 4.36
| 4.36
| 3.97
| 3.49
| 3.06
| 2.69
| 2.37
| 2.08
| 1.83
| 1.61
|
7.6
| 3.98
| 3.98
| 3.61
| 3.18
| 2.79
| 2.45
| 2.16
| 1.90
| 1.67
| 1.47
|
7.7
| 3.58
| 3.58
| 3.25
| 2.86
| 2.51
| 2.21
| 1.94
| 1.71
| 1.50
| 1.32
|
7.8
| 3.18
| 3.18
| 2.89
| 2.54
| 2.23
| 1.96
| 1.73
| 1.52
| 1.33
| 1.17
|
7.9
| 2.80
| 2.80
| 2.54
| 2.24
| 1.96
| 1.73
| 1.52
| 1.33
| 1.17
| 1.03
|
8.0
| 2.43
| 2.43
| 2.21
| 1.94
| 1.71
| 1.50
| 1.32
| 1.16
| 1.02
| 0.897
|
8.1
| 2.10
| 2.10
| 1.91
| 1.68
| 1.47
| 1.29
| 1.14
| 1.00
| 0.879
| 0.773
|
8.2
| 1.79
| 1.79
| 1.63
| 1.43
| 1.26
| 1.11
| 0.973
| 0.855
| 0.752
| 0.661
|
8.3
| 1.52
| 1.52
| 1.39
| 1.22
| 1.07
| 0.941
| 0.827
| 0.727
| 0.639
| 0.562
|
8.4
| 1.29
| 1.29
| 1.17
| 1.03
| 0.906
| 0.796
| 0.700
| 0.615
| 0.541
| 0.475
|
8.5
| 1.09
| 1.09
| 0.990
| 0.870
| 0.765
| 0.672
| 0.591
| 0.520
| 0.457
| 0.401
|
8.6
| 0.920
| 0.920
| 0.836
| 0.735
| 0.646
| 0.568
| 0.499
| 0.439
| 0.386
| 0.339
|
8.7
| 0.778
| 0.778
| 0.707
| 0.622
| 0.547
| 0.480
| 0.422
| 0.371
| 0.326
| 0.287
|
8.8
| 0.661
| 0.661
| 0.601
| 0.528
| 0.464
| 0.408
| 0.359
| 0.315
| 0.277
| 0.244
|
8.9
| 0.565
| 0.565
| 0.513
| 0.451
| 0.397
| 0.349
| 0.306
| 0.269
| 0.237
| 0.208
|
9.0
| 0.486
| 0.486
| 0.442
| 0.389
| 0.342
| 0.300
| 0.264
| 0.232
| 0.204
| 0.179
|

To calculate total ammonia nitrogen chronic criteria values in freshwater when fish freshwater mussels and early life stages of fish are present at different pH and temperature values than those listed in this subsection, use the following formulas equation and round the result to two significant digits:
Chronic Criteria Concentration =
(
| 0.0577
| +
| 2.487
| )
| x MIN
|
(1 + 107.688-pH)
| (1 + 10pH-7.688)
|
Where MIN = 2.85 or 1.45 x 100.028(25-T), whichever is less.
| 0.8876 X ( | 0.0278 | + | 1.1994 | ) X (2.126 X 100.028 X (20 - MAX(T,7))) |
| 1 + 107.688-pH | 1 + 10pH-7.688 |
Where MAX = 7 or temperature in degrees Celsius, whichever is greater
T = temperature in °C
2The default design flow for calculating steady state waste load allocations for the chronic ammonia criterion where early life stages of fish are present is the 30Q10 (see 9VAC25-260-140 B footnote 10) unless statistically valid methods are employed which demonstrate compliance with the duration and return frequency of the water quality criteria.
D. Site-specific considerations and alternate criteria. If it can be adequately demonstrated that freshwater mussels or early life stages of fish are not present at a site, then alternate site-specific criteria can be considered using the information provided in this subsection. Recalculated site-specific criteria shall provide for the attainment and maintenance of the water quality standards of downstream waters.
1. Site-specific modifications to the ambient water quality criteria for ammonia to account for the absence of freshwater mussels or early life stages of fish shall be conducted in accordance with the procedures contained in this subdivision. Because the department presumes that most state waterbodies have freshwater mussels and early life stages of fish present during most times of the year, the criteria shall be calculated assuming freshwater mussels and early life stages of fish are present using subsections B and C of this section unless the following demonstration that freshwater mussels or early life stages of fish are absent is successfully completed. Determination of the absence of freshwater mussels requires special field survey methods. This determination must be made after an adequate survey of the waterbody is conducted by an individual certified by the Virginia Department of Game and Inland Fisheries (DGIF) for freshwater mussel identification and surveys. Determination of absence of freshwater mussels will be done in consultation with the DGIF. Early life stages of fish are defined in subdivision 2 of this subsection. Modifications to the ambient water quality criteria for ammonia based on the presence or absence of early life stages of fish shall only apply at temperatures below 15°C.
a. During the review of any new or existing activity that has a potential to discharge ammonia in amounts that may cause or contribute to a violation of the ammonia criteria contained in subsection B of this section, the department may examine data from the following approved sources in subdivisions 1 a (1) through (5) of this subsection or may require the gathering of data in accordance with subdivisions 1 a (1) through (5) on the presence or absence of early life stages of fish in the affected waterbody.
(1) Species and distribution data contained in the Virginia Department of Game and Inland Fisheries Wildlife Information System database.
(2) Species and distribution data contained in Freshwater Fishes of Virginia, 1994.
(3) Data and fish species distribution maps contained in Handbook for Fishery Biology, Volume 3, 1997.
(4) Field data collected in accordance with U.S. EPA's Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers, Second Edition, EPA 841-B-99-002. Field data must comply with all quality assurance and quality control criteria.
(5) The American Society for Testing and Materials (ASTM) Standard E-1241-88, Standard Guide for Conducting Early Life-Stage Toxicity Tests with Fishes.
b. If data or information from sources other than subdivisions 1 a (1) through (5) of this subsection are considered, then any resulting site-specific criteria modifications shall be reviewed and adopted in accordance with the site-specific criteria provisions in 9VAC25-260-140 D and submitted to EPA for review and approval.
c. If the department determines that the data and information obtained from subdivisions 1 a (1) through (5) of this subsection demonstrate that there are periods of each year when no early life stages are expected to be present for any species of fish that occur at the site, the department shall issue a notice to the public and make available for public comment the supporting data and analysis along with the department's preliminary decision to authorize the site-specific modification to the ammonia criteria. Such information shall include, at a minimum:
(1) Sources of data and information.
(2) List of fish species that occur at the site as defined in subdivision 3 of this subsection.
(3) Definition of the site. Definition of a "site" can vary in geographic size from a stream segment to a watershed to an entire eco-region.
(4) Duration of early life stage for each species in subdivision 1 c (2) of this subsection.
(5) Dates when early life stages of fish are expected to be present for each species in subdivision 1 c (2) of this subsection.
(6) Based on subdivision 1 c (5) of this subsection, identify the dates (beginning date, ending date), if any, where no early life stages are expected to be present for any of the species identified in subdivision 1 c (2) of this subsection.
d. If, after reviewing the public comments received in subdivision 1 c of this subsection and supporting data and information, the department determines that there are times of the year when no early life stages are expected to be present for any fish species that occur at the site, then the applicable ambient water quality criteria for ammonia for those time periods shall be calculated using the table in this subsection, or the formula for calculating the chronic criterion concentration for ammonia when early life stages of fish are absent.
e. The department shall maintain a comprehensive list of all sites where the department has determined that early life stages of fish are absent. For each site the list will identify the waterbodies affected and the corresponding times of the year that early life stages of fish are absent. This list is available either upon request from the Office of Water Quality Programs at 629 East Main Street, Richmond, VA 23219, or from the department website at http://www.deq.virginia.gov/programs/water/waterqualityinformationtmdls/waterqualitystandards.aspx.
2. The duration of the "early life stages" extends from the beginning of spawning through the end of the early life stages. The early life stages include the prehatch embryonic period, the post-hatch free embryo or yolk-sac fry, and the larval period, during which the organism feeds. Juvenile fish, which are anatomically similar to adults, are not considered an early life stage. The duration of early life stages can vary according to fish species. The department considers the sources of information in subdivisions 1 a (1) through (5) of this subsection to be the only acceptable sources of information for determining the duration of early life stages of fish under this procedure.
3. "Occur at the site" includes the species, genera, families, orders, classes, and phyla that are usually present at the site; are present at the site only seasonally due to migration; are present intermittently because they periodically return to or extend their ranges into the site; or were present at the site in the past or are present in nearby bodies of water, but are not currently present at the site due to degraded conditions, and are expected to return to the site when conditions improve. "Occur at the site" does not include taxa that were once present at the site but cannot exist at the site now due to permanent physical alteration of the habitat at the site.
4. Any modifications to ambient water quality criteria for ammonia in subdivision 1 of this subsection shall not likely jeopardize the continued existence of any federal or state listed, threatened, or endangered species or result in the destruction or adverse modification of such species' critical habitats.
5. Site-specific modifications to the ambient water quality criteria for ammonia to account for the absence of freshwater mussels shall be conducted in accordance with the procedures contained in this subsection. Because the department presumes that most state waterbodies have freshwater mussel species, the criteria shall be calculated assuming mussels are present using subsections B and C of this section unless the demonstration that freshwater mussels are absent is successfully completed and accepted by DEQ and DGIF.
6. Equations for calculating ammonia criteria for four different site-specific scenarios are provided in subdivisions a through d of this subdivision 6 as follows: (i) acute criteria when mussels are absent but trout are present, (ii) acute criteria when mussels and trout are absent, (iii) chronic criteria when mussels are absent and early life stages of fish are present, and (iv) chronic criteria when mussels and early life stages of fish are absent. Additional information regarding site-specific criteria can be reviewed in appendix N (pages 225-242) of the EPA Aquatic Life Ambient Water Quality Criteria to Ammonia--Freshwater 2013 (EPA 822-R-13-001).
a. Acute criteria: freshwater mussels absent and trout present. To calculate total ammonia nitrogen acute criteria values (in mg N/L) in freshwater with freshwater mussels absent (procedures for making this determination are in subdivisions 1 through 5 of this subsection) and trout present, use the following equations. The acute criterion is the lesser of the calculation results below. Round the result to two significant digits.
| ( | 0.275 | + | 39 | ) |
| 1 + 107.204-pH | 1 + 10pH-7.204 |
or
| 0.7249 X ( | 0.0114 | + | 1.6181 | ) X (62.15 X 100.036X(20 – T)) |
| 1 + 107.204-pH | 1 + 10pH-7.204 |
b. Acute criteria: freshwater mussels absent and trout absent. To calculate total ammonia nitrogen acute criteria values (in mg N/L) in freshwater where freshwater mussels are absent and trout are absent, use the following equation. Round the result to two significant digits.
| 0.7249 X ( | 0.0114 | + | 1.6181 | ) X MIN |
| 1 + 107.204-pH | 1 + 10pH-7.204 |
Where MIN = 51.93 or 62.15 X 100.036 X (20 – T), whichever is less
T = Temperature in oC
C. The 30-day average concentration of c. Chronic criteria: freshwater mussels absent and early life stages of fish present. The chronic criteria for total ammonia nitrogen (in mg N/L) where early life stages of fish freshwater mussels are absent (procedures for making this determination are in subdivisions 1 through 4 5 of this subsection) in freshwater shall not exceed, more than once every three years on the average3, the chronic criteria below: concentration values calculated using the following equation. Round the result to two significant digits.
Chronic Ammonia Freshwater Criteria
Early Life Stages of Fish Absent
Total Ammonia Nitrogen (mg N/L)
|
| Temperature (°C)
|
pH
| 0-7
| 8
| 9
| 10
| 11
| 12
| 13
| 14
| 15
| 16
|
6.5
| 10.8
| 10.1
| 9.51
| 8.92
| 8.36
| 7.84
| 7.35
| 6.89
| 6.46
| 6.06
|
6.6
| 10.7
| 9.99
| 9.37
| 8.79
| 8.24
| 7.72
| 7.24
| 6.79
| 6.36
| 5.97
|
6.7
| 10.5
| 9.81
| 9.20
| 8.62
| 8.08
| 7.58
| 7.11
| 6.66
| 6.25
| 5.86
|
6.8
| 10.2
| 9.58
| 8.98
| 8.42
| 7.90
| 7.40
| 6.94
| 6.51
| 6.10
| 5.72
|
6.9
| 9.93
| 9.31
| 8.73
| 8.19
| 7.68
| 7.20
| 6.75
| 6.33
| 5.93
| 5.56
|
7.0
| 9.60
| 9.00
| 8.43
| 7.91
| 7.41
| 6.95
| 6.52
| 6.11
| 5.73
| 5.37
|
7.1
| 9.20
| 8.63
| 8.09
| 7.58
| 7.11
| 6.67
| 6.25
| 5.86
| 5.49
| 5.15
|
7.2
| 8.75
| 8.20
| 7.69
| 7.21
| 6.76
| 6.34
| 5.94
| 5.57
| 5.22
| 4.90
|
7.3
| 8.24
| 7.73
| 7.25
| 6.79
| 6.37
| 5.97
| 5.60
| 5.25
| 4.92
| 4.61
|
7.4
| 7.69
| 7.21
| 6.76
| 6.33
| 5.94
| 5.57
| 5.22
| 4.89
| 4.59
| 4.30
|
7.5
| 7.09
| 6.64
| 6.23
| 5.84
| 5.48
| 5.13
| 4.81
| 4.51
| 4.23
| 3.97
|
7.6
| 6.46
| 6.05
| 5.67
| 5.32
| 4.99
| 4.68
| 4.38
| 4.11
| 3.85
| 3.61
|
7.7
| 5.81
| 5.45
| 5.11
| 4.79
| 4.49
| 4.21
| 3.95
| 3.70
| 3.47
| 3.25
|
7.8
| 5.17
| 4.84
| 4.54
| 4.26
| 3.99
| 3.74
| 3.51
| 3.29
| 3.09
| 2.89
|
7.9
| 4.54
| 4.26
| 3.99
| 3.74
| 3.51
| 3.29
| 3.09
| 2.89
| 2.71
| 2.54
|
8.0
| 3.95
| 3.70
| 3.47
| 3.26
| 3.05
| 2.86
| 2.68
| 2.52
| 2.36
| 2.21
|
8.1
| 3.41
| 3.19
| 2.99
| 2.81
| 2.63
| 2.47
| 2.31
| 2.17
| 2.03
| 1.91
|
8.2
| 2.91
| 2.73
| 2.56
| 2.40
| 2.25
| 2.11
| 1.98
| 1.85
| 1.74
| 1.63
|
8.3
| 2.47
| 2.32
| 2.18
| 2.04
| 1.91
| 1.79
| 1.68
| 1.58
| 1.48
| 1.39
|
8.4
| 2.09
| 1.96
| 1.84
| 1.73
| 1.62
| 1.52
| 1.42
| 1.33
| 1.25
| 1.17
|
8.5
| 1.77
| 1.66
| 1.55
| 1.46
| 1.37
| 1.28
| 1.20
| 1.13
| 1.06
| 0.990
|
8.6
| 1.49
| 1.40
| 1.31
| 1.23
| 1.15
| 1.08
| 1.01
| 0.951
| 0.892
| 0.836
|
8.7
| 1.26
| 1.18
| 1.11
| 1.04
| 0.976
| 0.915
| 0.858
| 0.805
| 0.754
| 0.707
|
8.8
| 1.07
| 1.01
| 0.944
| 0.885
| 0.829
| 0.778
| 0.729
| 0.684
| 0.641
| 0.601
|
8.9
| 0.917
| 0.860
| 0.806
| 0.756
| 0.709
| 0.664
| 0.623
| 0.584
| 0.548
| 0.513
|
9.0
| 0.790
| 0.740
| 0.694
| 0.651
| 0.610
| 0.572
| 0.536
| 0.503
| 0.471
| 0.442
|
At 15°C and above, the criterion for fish early life stages absent is the same as the criterion for fish early life stages present.
To calculate total ammonia nitrogen chronic criteria values in freshwater when fish early life stages are absent at different pH and temperature values than those listed in this subsection, use the following formulas:
Chronic Criteria Concentration =
(
| 0.0577
| +
| 2.487
| )
| x 1.45(100.028(25-MAX))
|
(1 + 107.688-pH)
| (1 + 10pH-7.688)
|
MAX = temperature in °C or 7, whichever is greater.
3The default design flow for calculating steady state waste load allocations for the chronic ammonia criterion where early life stages of fish are absent is the 30Q10 (see 9VAC25-260-140 B footnote 10) unless statistically valid methods are employed that demonstrate compliance with the duration and return frequency of the water quality criteria.
1. Site-specific modifications to the ambient water quality criteria for ammonia to account for the absence of early life stages of fish shall be conducted in accordance with the procedures contained in this subdivision. Because the department presumes that most state waterbodies have early life stages of fish present during most times of the year, the criteria shall be calculated assuming early life stages of fish are present using subsection B of this section unless the following demonstration that early life stages are absent is successfully completed. Early life stages of fish are defined in subdivision 2 of this subsection. Modifications to the ambient water quality criteria for ammonia based on the presence or absence of early life stages of fish shall only apply at temperatures below 15°C.
a. During the review of any new or existing activity that has a potential to discharge ammonia in amounts that may cause or contribute to a violation of the ammonia criteria contained in subsection B of this section, the department may examine data from the following approved sources in subdivisions 1 a (1) through (5) of this subsection or may require the gathering of data in accordance with subdivisions 1 a (1) through (5) on the presence or absence of early life stages of fish in the affected waterbody.
(1) Species and distribution data contained in the Virginia Department of Game and Inland Fisheries Wildlife Information System database.
(2) Species and distribution data contained in Freshwater Fishes of Virginia, 1994.
(3) Data and fish species distribution maps contained in Handbook for Fishery Biology, Volume 3, 1997.
(4) Field data collected in accordance with U.S. EPA's Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers, Second Edition, EPA 841-B-99-002. Field data must comply with all quality assurance/quality control criteria.
(5) The American Society for Testing and Materials (ASTM) Standard E-1241-88, Standard Guide for Conducting Early Life-Stage Toxicity Tests with Fishes.
b. If data or information from sources other than subdivisions 1 a (1) through (5) of this subsection are considered, then any resulting site-specific criteria modifications shall be reviewed and adopted in accordance with the site-specific criteria provisions in 9VAC25-260-140 D, and submitted to EPA for review and approval.
c. If the department determines that the data and information obtained from subdivisions 1 a (1) through (5) of this subsection demonstrate that there are periods of each year when no early life stages are expected to be present for any species of fish that occur at the site, the department shall issue a notice to the public and make available for public comment the supporting data and analysis along with the department's preliminary decision to authorize the site-specific modification to the ammonia criteria. Such information shall include, at a minimum:
(1) Sources of data and information.
(2) List of fish species that occur at the site as defined by subdivision 3 of this subsection.
(3) Definition of the site. Definition of a "site" can vary in geographic size from a stream segment to a watershed to an entire eco-region.
(4) Duration of early life stage for each species in subdivision 1 c (2) of this subsection.
(5) Dates when early life stages of fish are expected to be present for each species in subdivision 1 c (2) of this subsection.
(6) Based on subdivision 1 c (5) of this subsection, identify the dates (beginning date, ending date), if any, where no early life stages are expected to be present for any of the species identified in subdivision 1 c (2) of this subsection.
d. If, after reviewing the public comments received in subdivision 1 c of this subsection and supporting data and information, the department determines that there are times of the year where no early life stages are expected to be present for any fish species that occur at the site, then the applicable ambient water quality criteria for ammonia for those time periods shall be calculated using the table in this subsection, or the formula for calculating the chronic criterion concentration for ammonia when fish early life stages are absent.
e. The department shall maintain a comprehensive list of all sites where the department has determined that early life stages of fish are absent. For each site the list will identify the waterbodies affected and the corresponding times of the year that early life stages are absent. This list is available either upon request from the Office of Water Quality Programs at P.O. Box 1105, Richmond, Virginia 23218 or from the department website http://www.deq.virginia.gov/wqs.
2. The duration of the "early life stages" extends from the beginning of spawning through the end of the early life stages. The early life stages include the prehatch embryonic period, the post-hatch free embryo or yolk-sac fry, and the larval period, during which the organism feeds. Juvenile fish, which are anatomically similar to adults, are not considered an early life stage. The duration of early life stages can vary according to fish species. The department considers the sources of information in subdivisions 1 a (1) through (5) of this subsection to be the only acceptable sources of information for determining the duration of early life stages of fish under this procedure.
3. "Occur at the site" includes the species, genera, families, orders, classes, and phyla that: are usually present at the site; are present at the site only seasonally due to migration; are present intermittently because they periodically return to or extend their ranges into the site; were present at the site in the past or are present in nearby bodies of water, but are not currently present at the site due to degraded conditions, and are expected to return to the site when conditions improve. "Occur at the site" does not include taxa that were once present at the site but cannot exist at the site now due to permanent physical alteration of the habitat at the site.
4. Any modifications to ambient water quality criteria for ammonia in subdivision 1 of this subsection shall not likely jeopardize the continued existence of any federal or state listed, threatened or endangered species or result in the destruction or adverse modification of such species' critical habitat.
| 0.9405 X ( | 0.0278 | + | 1.1994 | ) X MIN |
| 1 + 107.688-pH | 1 + 10pH-7.688 |
Where MIN = 6.920 or 7.547 X 100.028 x (20 – T) whichever is less
T = temperature in °C
d. Chronic criteria: freshwater mussels absent and early life stages of fish absent. The chronic criteria for total ammonia nitrogen (in mg N/L) where freshwater mussels are absent and early life stages of fish are absent (procedures for making this determination are in subdivisions 1 through 5 of this subsection) in freshwater shall not exceed concentration values calculated using the following equation. Round the result to two significant digits.
| 0.9405 X ( | 0.0278 | + | 1.1994 | ) X(7.547 X 100.028 X (20 - MAX(T,7))) |
| 1 + 107.688-pH | 1 + 10pH-7.688 |
Where MAX = 7 or temperature in degrees Celsius, whichever is greater
T = temperature in °C
D. E. The one-hour average concentration of total ammonia nitrogen (in mg N/L) in saltwater shall not exceed, more than once every three years on the average, the acute criteria below:
Acute Ammonia Saltwater Criteria
Total Ammonia Nitrogen (mg N/L)
Salinity = 10 g/kg |
| Temperature °C |
pH | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 |
7.00 | 231.9 | 159.8 | 110.1 | 75.88 | 52.31 | 36.08 | 24.91 | 17.21 |
7.20 | 146.4 | 100.9 | 69.54 | 47.95 | 33.08 | 22.84 | 15.79 | 10.93 |
7.40 | 92.45 | 63.73 | 43.94 | 30.32 | 20.94 | 14.48 | 10.03 | 6.97 |
7.60 | 58.40 | 40.28 | 27.80 | 19.20 | 13.28 | 9.21 | 6.40 | 4.47 |
7.80 | 36.92 | 25.48 | 17.61 | 12.19 | 8.45 | 5.88 | 4.11 | 2.89 |
8.00 | 23.37 | 16.15 | 11.18 | 7.76 | 5.40 | 3.78 | 2.66 | 1.89 |
8.20 | 14.81 | 10.26 | 7.13 | 4.97 | 3.48 | 2.46 | 1.75 | 1.27 |
8.40 | 9.42 | 6.54 | 4.57 | 3.20 | 2.27 | 1.62 | 1.18 | 0.87 |
8.60 | 6.01 | 4.20 | 2.95 | 2.09 | 1.50 | 1.09 | 0.81 | 0.62 |
8.80 | 3.86 | 2.72 | 1.93 | 1.39 | 1.02 | 0.76 | 0.58 | 0.46 |
9.00 | 2.51 | 1.79 | 1.29 | 0.95 | 0.71 | 0.55 | 0.44 | 0.36 |
Salinity = 20 g/kg |
| Temperature °C |
pH | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 |
7.00 | 247.6 | 170.5 | 117.5 | 80.98 | 55.83 | 38.51 | 26.58 | 18.36 |
7.20 | 156.3 | 107.7 | 74.21 | 51.17 | 35.30 | 24.37 | 16.84 | 11.66 |
7.40 | 98.67 | 68.01 | 46.90 | 32.35 | 22.34 | 15.44 | 10.70 | 7.43 |
7.60 | 62.33 | 42.98 | 29.66 | 20.48 | 14.17 | 9.82 | 6.82 | 4.76 |
7.80 | 39.40 | 27.19 | 18.78 | 13.00 | 9.01 | 6.26 | 4.37 | 3.07 |
8.00 | 24.93 | 17.23 | 11.92 | 8.27 | 5.76 | 4.02 | 2.83 | 2.01 |
8.20 | 15.80 | 10.94 | 7.59 | 5.29 | 3.70 | 2.61 | 1.86 | 1.34 |
8.40 | 10.04 | 6.97 | 4.86 | 3.41 | 2.41 | 1.72 | 1.24 | 0.91 |
8.60 | 6.41 | 4.47 | 3.14 | 2.22 | 1.59 | 1.15 | 0.85 | 0.65 |
8.80 | 4.11 | 2.89 | 2.05 | 1.47 | 1.07 | 0.80 | 0.61 | 0.48 |
9.00 | 2.67 | 1.90 | 1.36 | 1.00 | 0.75 | 0.57 | 0.46 | 0.37 |
Salinity = 30 g/kg |
| Temperature °C |
pH | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 |
7.00 | 264.6 | 182.3 | 125.6 | 86.55 | 59.66 | 41.15 | 28.39 | 19.61 |
7.20 | 167.0 | 115.1 | 79.31 | 54.68 | 37.71 | 26.03 | 17.99 | 12.45 |
7.40 | 105.5 | 72.68 | 50.11 | 34.57 | 23.87 | 16.50 | 11.42 | 7.92 |
7.60 | 66.61 | 45.93 | 31.69 | 21.88 | 15.13 | 10.48 | 7.28 | 5.07 |
7.80 | 42.10 | 29.05 | 20.07 | 13.88 | 9.62 | 6.68 | 4.66 | 3.27 |
8.00 | 26.63 | 18.40 | 12.73 | 8.83 | 6.14 | 4.29 | 3.01 | 2.13 |
8.20 | 16.88 | 11.68 | 8.10 | 5.64 | 3.94 | 2.78 | 1.97 | 1.42 |
8.40 | 10.72 | 7.44 | 5.18 | 3.63 | 2.56 | 1.82 | 1.31 | 0.96 |
8.60 | 6.83 | 4.77 | 3.34 | 2.36 | 1.69 | 1.22 | 0.90 | 0.68 |
8.80 | 4.38 | 3.08 | 2.18 | 1.56 | 1.13 | 0.84 | 0.64 | 0.50 |
9.00 | 2.84 | 2.01 | 1.45 | 1.06 | 0.79 | 0.60 | 0.47 | 0.39 |
| | | | | | | | | | | | | | | | |
To calculate total ammonia nitrogen acute criteria values in saltwater at different pH and temperature values than those listed in this subsection, use the following formulas:
I = | 19.9273S |
(1000 - 1.005109S) |
Where I = molal ionic strength of water
S = Salinity ppt (g/kg)
The regression model used to relate I to pKa (negative log of the ionization constant) is
pKa = 9.245 + 0.138(I)
pKa as defined by these equations is at 298 degrees Kelvin (25°C). T °Kelvin = °C + 273
To correct for other temperatures:
pKaST = pKaS298 + 0.0324(298 - T °Kelvin)
The unionized ammonia fraction (UIA) is given by:
The acute ammonia criterion in saltwater is given by:
Multiply the acute value by 0.822 to get the ammonia-N acute criterion.
E. F. The 30-day average concentration of total ammonia nitrogen (in mg N/L) in saltwater shall not exceed, more than once every three years on the average, the chronic criteria below:
Chronic Ammonia Saltwater Criteria
Total Ammonia Nitrogen (mg N/L)
Salinity = 10 g/kg |
| Temperature °C |
pH | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 |
7.00 | 34.84 | 24.00 | 16.54 | 11.40 | 7.86 | 5.42 | 3.74 | 2.59 |
7.20 | 21.99 | 15.15 | 10.45 | 7.20 | 4.97 | 3.43 | 2.37 | 1.64 |
7.40 | 13.89 | 9.57 | 6.60 | 4.55 | 3.15 | 2.18 | 1.51 | 1.05 |
7.60 | 8.77 | 6.05 | 4.18 | 2.88 | 2.00 | 1.38 | 0.96 | 0.67 |
7.80 | 5.55 | 3.83 | 2.65 | 1.83 | 1.27 | 0.88 | 0.62 | 0.43 |
8.00 | 3.51 | 2.43 | 1.68 | 1.17 | 0.81 | 0.57 | 0.40 | 0.28 |
8.20 | 2.23 | 1.54 | 1.07 | 0.75 | 0.52 | 0.37 | 0.26 | 0.19 |
8.40 | 1.41 | 0.98 | 0.69 | 0.48 | 0.34 | 0.24 | 0.18 | 0.13 |
8.60 | 0.90 | 0.63 | 0.44 | 0.31 | 0.23 | 0.16 | 0.12 | 0.09 |
8.80 | 0.58 | 0.41 | 0.29 | 0.21 | 0.15 | 0.11 | 0.09 | 0.07 |
9.00 | 0.38 | 0.27 | 0.19 | 0.14 | 0.11 | 0.08 | 0.07 | 0.05 |
Salinity = 20 g/kg | |
| Temperature °C | |
pH | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | |
7.00 | 37.19 | 25.62 | 17.65 | 12.16 | 8.39 | 5.78 | 3.99 | 2.76 | |
7.20 | 23.47 | 16.17 | 11.15 | 7.69 | 5.30 | 3.66 | 2.53 | 1.75 | |
7.40 | 14.82 | 10.22 | 7.04 | 4.86 | 3.36 | 2.32 | 1.61 | 1.12 | |
7.60 | 9.36 | 6.46 | 4.46 | 3.08 | 2.13 | 1.47 | 1.02 | 0.71 | |
7.80 | 5.92 | 4.08 | 2.82 | 1.95 | 1.35 | 0.94 | 0.66 | 0.46 | |
8.00 | 3.74 | 2.59 | 1.79 | 1.24 | 0.86 | 0.60 | 0.43 | 0.30 | |
8.20 | 2.37 | 1.64 | 1.14 | 0.79 | 0.56 | 0.39 | 0.28 | 0.20 | |
8.40 | 1.51 | 1.05 | 0.73 | 0.51 | 0.36 | 0.26 | 0.19 | 0.14 | |
8.60 | 0.96 | 0.67 | 0.47 | 0.33 | 0.24 | 0.17 | 0.13 | 0.10 | |
8.80 | 0.62 | 0.43 | 0.31 | 0.22 | 0.16 | 0.12 | 0.09 | 0.07 | |
9.00 | 0.40 | 0.28 | 0.20 | 0.15 | 0.11 | 0.09 | 0.07 | 0.06 | |
Salinity = 30 g/kg | |
| Temperature °C | |
pH | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | |
7.00 | 39.75 | 27.38 | 18.87 | 13.00 | 8.96 | 6.18 | 4.27 | 2.95 | |
7.20 | 25.09 | 17.29 | 11.91 | 8.21 | 5.67 | 3.91 | 2.70 | 1.87 | |
7.40 | 15.84 | 10.92 | 7.53 | 5.19 | 3.59 | 2.48 | 1.72 | 1.19 | |
7.60 | 10.01 | 6.90 | 4.76 | 3.29 | 2.27 | 1.57 | 1.09 | 0.76 | |
7.80 | 6.32 | 4.36 | 3.01 | 2.08 | 1.44 | 1.00 | 0.70 | 0.49 | |
8.00 | 4.00 | 2.76 | 1.91 | 1.33 | 0.92 | 0.64 | 0.45 | 0.32 | |
8.20 | 2.53 | 1.75 | 1.22 | 0.85 | 0.59 | 0.42 | 0.30 | 0.21 | |
8.40 | 1.61 | 1.12 | 0.78 | 0.55 | 0.38 | 0.27 | 0.20 | 0.14 | |
8.60 | 1.03 | 0.72 | 0.50 | 0.35 | 0.25 | 0.18 | 0.14 | 0.10 | |
8.80 | 0.66 | 0.46 | 0.33 | 0.23 | 0.17 | 0.13 | 0.10 | 0.08 | |
9.00 | 0.43 | 0.30 | 0.22 | 0.16 | 0.12 | 0.09 | 0.07 | 0.06 | |
| | | | | | | | | | | | | | | | | | | | | | | |
To calculate total ammonia nitrogen chronic criteria values in saltwater at different pH and temperature values than those listed in this subsection, use the following formulas:
I = | 19.9273S |
(1000 - 1.005109S) |
Where I = molal ionic strength of water
S = Salinity ppt (g/kg)
The regression model used to relate I to pKa (negative log of the ionization constant) is
pKa = 9.245 + 0.138(I)
pKa as defined by these equations is at 298 degrees Kelvin (25°C). T °Kelvin = °C + 273
To correct for other temperatures:
pKaST = pKaS298 + 0.0324(298 - T °Kelvin)
The unionized ammonia fraction (UIA) is given by:
The chronic ammonia criterion in saltwater is given by:
Multiply the chronic value by 0.822 to get the ammonia-N chronic criterion.
1The default design flow for calculating steady state wasteload allocations for the acute ammonia criterion for freshwater is the 1Q10 (see 9VAC25-260-140 B footnote 10) unless statistically valid methods are employed which demonstrate compliance with the duration and return frequency of the water quality criteria.
2The default design flow for calculating steady state wasteload allocations for the chronic ammonia criterion for freshwater is the 30Q10 (see 9VAC25-260-140 B footnote 10) unless statistically valid methods are employed which demonstrate compliance with the duration and return frequency of the water quality criteria.
G. Implementation of ammonia criteria through Virginia Pollutant Discharge Elimination System (VPDES) Permits. The ammonia criteria in subsections A, B, and C of this section shall be addressed during individual VPDES permit reissuance for existing dischargers subject to new or more restrictive water quality-based ammonia effluent limits in accordance with the department's standard permitting practices except as follows:
1. Notwithstanding any other regulatory requirement, a compliance schedule may be established that exceeds the term of the permit, subject to a demonstration by the permittee that a longer period is necessary to allow a reasonable opportunity to attain compliance with the new or more restrictive ammonia discharge requirements. The department's consideration for such a demonstration shall be made on a case-by-case basis and shall require compliance as soon as possible, but not later than the applicable statutory deadline under the Clean Water Act.
2. Information to be provided under subdivision 1 of this subsection may include such factors as (i) opportunities to minimize costs to the public or facility owners by phasing in the implementation of multiple projects, (ii) time needed for freshwater mussel habitat determinations, and (iii) other relevant factors.
3. If a permit establishes a schedule of compliance that exceeds the term of the permit, the compliance schedule shall set forth interim requirements and the dates for their achievement.
a. The time between interim dates shall not exceed one year.
b. If the time necessary for completion of any interim requirement is more than one year and is not readily divisible into stages for completion, the permit shall specify interim dates for the submission of reports of progress toward completion of the interim requirements and indicate a projected completion date.
c. The permit shall be written to require that no later than 14 days following each interim date and the final date of compliance, the permittee shall notify the department in writing of its compliance or noncompliance with the interim or final requirements, or submit progress reports if subdivision 3 b of this subsection is applicable.
d. Any change to an interim compliance date in the schedule of compliance will be deemed to be a minor modification of the permit, provided the new date is not more than 120 days after the date specified in the existing permit and does not interfere with attainment of the final compliance date requirement.
9VAC25-260-170. Bacteria; other recreational waters.
A. The following bacteria criteria (colony forming units (CFU)/100 ml) shall apply to protect primary contact recreational uses in surface waters, except waters identified in subsection B of this section:
E. coli bacteria shall not exceed a monthly geometric mean of 126 CFU/100 ml in freshwater and no more than 10% of the samples in the assessment period shall exceed a statistical threshold value (STV) of 410 CFU/100 ml.
Enterococci bacteria shall not exceed a monthly geometric mean of 35 CFU/100 ml in transition and saltwater and no more than 10% of the samples in the assessment period shall exceed a statistical threshold value (STV) of 130 CFU/100 ml.
1. See 9VAC25-260-140 C for boundary delineations for freshwater, transition and saltwater.
2. Geometric means shall be calculated using all data collected during any calendar month with a minimum of four weekly samples. The Virginia Department of Health shall make determinations regarding beach advisories or closures.
3. If there are insufficient data to calculate monthly geometric means in freshwater, no more than 10% of the total samples in the assessment period shall exceed 235 E. coli CFU/100 ml.
4. If there are insufficient data to calculate monthly geometric means in transition and saltwater, no more than 10% of the total samples in the assessment period shall exceed enterococci 104 CFU/100 ml.
5. For beach advisories or closures, a single sample maximum of 235 E. coli CFU/100 ml in freshwater and a single sample maximum of 104 enterococci CFU/100 ml in saltwater and transition zones shall apply .
B. The following bacteria criteria per 100 ml (CFU/100 ml) of water shall apply:
E. coli bacteria shall not exceed a monthly geometric mean of 630 CFU/100 ml in freshwater.
Enterococci bacteria shall not exceed a monthly geometric mean of 175 CFU/100 ml in transition and saltwater.
1. See 9VAC25-260-140 C for boundary delineations for freshwater, transition and saltwater.
2. Geometric means shall be calculated using all data collected during any calendar month with a minimum of four weekly samples.
3. If there is insufficient data to calculate monthly geometric means in freshwater, no more than 10% of the total samples in the assessment period shall exceed 1173 E. coli CFU/100 ml.
4. If there is insufficient data to calculate monthly geometric means in transition and saltwater, no more than 10% of the total samples in the assessment period shall exceed 519 enterococci CFU/100 ml.
5. Where the existing water quality for bacteria is below the geometric mean criteria in a water body designated for secondary contact in subdivision 6 of this subsection that higher water quality will be maintained in accordance with 9VAC25-260-30 A 2.
6. Surface waters designated under this subsection are as follows:
a. (Reserved)
b. (Reserved)
c. (Reserved)
VA.R. Doc. No. R18-2148; Filed August 18, 2017, 10:09 a.m.
TITLE 11. GAMING
VIRGINIA RACING COMMISSION
Final Regulation
REGISTRAR'S NOTICE: The
Virginia Racing Commission is claiming an exemption from the Administrative
Process Act pursuant to § 2.2-4002 A 17 of the Code of Virginia (i) when
acting by and through its duly appointed stewards or in matters related to any
specific race meeting or (ii) in promulgating technical rules regulating actual
live horse racing at race meetings licensed by the commission.
Title of Regulation: 11VAC10-150. Harness Racing (amending 11VAC10-150-190).
Statutory Authority: § 59.1-369 of the Code of Virginia.
Effective Date: September 16, 2017.
Agency Contact: David S. Lermond, Jr., Regulatory
Coordinator, Virginia Racing Commission, 5707 Huntsman Road, Suite 201-B,
Richmond, VA 23250, telephone (804) 966-7404, or email
david.lermond@vrc.virginia.gov.
Summary:
The amendments authorize the stewards to extend the
requirement for a harness horse having to qualify for a particular race or race
meet from 30 days to 45 days.
11VAC10-150-190. Qualifying races.
A. No Standardbred may be raced unless it has a race
at the chosen gait, with a charted line in qualifying time, within 30 days of
its last race; however, this may be extended to 45 days for a particular
race or race meet with the approval of the stewards.
B. If a Standardbred does not have a charted line
within 30 days (or 45 days if approved by the stewards in accordance with
subsection A of this section) of its race, then the horse must race in a
qualifying race under the supervision of the stewards to determine its fitness
for racing.
C. The following provisions shall apply to qualifying
races:
1. The licensee shall provide appropriate personnel for
qualifying races to keep a charted line for each Standardbred in each
qualifying race, an electronic timing device shall be in operation, and a
photo-finish camera shall be in operation;
2. The licensee shall schedule as many qualifying races on as
many days as is deemed appropriate for the horse supply, and the licensee shall
maintain the racing surface in condition so that all Standardbreds have a
reasonable opportunity to meet the qualifying time;
3. A Standardbred must race in a qualifying race if it has one
race over a fast track that is not in the qualifying time as agreed upon by the
licensee and the representative of the horsemen or on gait;
4. A Standardbred coming off the Veterinarian's List must race
in a qualifying race, and the stewards, in their discretion, may require the
horse to race in one or more qualifying races to establish its fitness for
racing; and
5. The stewards, in their discretion, may authorize the
collection of blood, urine or other samples of body substances from
Standardbreds after competing in qualifying races.
VA.R. Doc. No. R18-5215; Filed August 21, 2017, 1:49 p.m.
TITLE 12. HEALTH
STATE BOARD OF HEALTH
Final Regulation
REGISTRAR'S NOTICE: The following regulatory action is exempt from Article 2 of the Administrative Process Act in accordance with § 2.2-4006 A 4 c of the Code of Virginia, which excludes regulations that are necessary to meet the requirements of federal law or regulations, provided such regulations do not differ materially from those required by federal law or regulation. The State Board of Health will receive, consider, and respond to petitions by any interested person at any time with respect to reconsideration or revision.
Title of Regulation: 12VAC5-481. Virginia Radiation Protection Regulations (amending 12VAC5-481-10, 12VAC5-481-421, 12VAC5-481-451, 12VAC5-481-1110, 12VAC5-481-2970, 12VAC5-481-3000, 12VAC5-481-3030, 12VAC5-481-3070, 12VAC5-481-3100, 12VAC5-481-3130, 12VAC5-481-3770).
Statutory Authority: § 32.1-229 of the Code of Virginia.
Effective Date: October 18, 2017.
Agency Contact: Steve Harrison, Director, Office of Radiological Health, Virginia Department of Health, 109 Governor Street, Richmond, VA 23219, telephone (804) 864-8151, FAX (804) 864-8155, or email steve.harrison@vdh.virginia.gov.
Summary:
The purpose of this final exempt action is to amend the Radiation Protection Regulations to ensure their compatibility with Title 10 of the Code of Federal Regulations. The amendments implement changes to regulations adopted by the Nuclear Regulatory Commission (NRC) in 2015. The amendments (i) relate to reportable safety events involving special nuclear material; (ii) remove the Safeguards Information – Modified Handling (SGI-M) designation of the security-related information for large irradiators, manufacturers and distributors, and transport of category 1 quantities of radioactive material; (iii) make updates for the packaging and transportation of radioactive material based on the International Atomic Energy Agency's 2009 standards for the international transportation of radioactive material and maintain consistency with the federal Department of Transportation's regulations; and (iv) correct references, typographical errors, and misspellings.
Part I
General Provisions
12VAC5-481-10. Definitions.
The following words and terms as used in this chapter shall have the following meanings unless the context clearly indicates otherwise:
"A1" means the maximum activity of special form radioactive material permitted in a Type A package. This value is listed in Table 1 of 12VAC5-481-3770 F.
"A2" means the maximum activity of radioactive material, other than special form radioactive material, LSA, and SCO material, permitted in a Type A package. This value is listed in Table 1 of 12VAC5-481-3770 F.
"Absorbed dose" means the energy imparted by ionizing radiation per unit mass of irradiated material. The units of absorbed dose are the gray (Gy) and the rad.
"Absorbed dose rate" means absorbed dose per unit time, for machines with timers, or dose monitor unit per unit time for linear accelerators.
"Accelerator" means any machine capable of accelerating electrons, protons, deuterons, or other charged particles in a vacuum and of discharging the resultant particulate or other radiation into a medium at energies usually in excess of one MeV. For purposes of this definition, "particle accelerator" is an equivalent term.
"Accelerator-produced material" means any material made radioactive by a particle accelerator.
"Access control" means a system for allowing only approved individuals to have unescorted access to the security zone and for ensuring that all other individuals are subject to escorted access.
"Accessible surface" means the external surface of the enclosure or housing of the radiation producing machine as provided by the manufacturer. It also means surface of equipment or of an equipment part that can be easily or accidentally touched by persons without the use of a tool.
"Act" means §§ 32.1-227 through 32.1-238 of the Code of Virginia.
"Active maintenance" means any significant activity needed during the period of institutional control to maintain a reasonable assurance that the performance objectives in 12VAC5-481-2490 and 12VAC5-481-2500 are met. Such active maintenance includes ongoing activities such as the pumping and treatment of water from a disposal unit or one-time measures such as replacement of a disposal unit cover. Active maintenance does not include custodial activities such as repair of fencing, repair or replacement of monitoring equipment, revegetation, minor additions to soil cover, minor repair of disposal unit covers, and general disposal site upkeep such as mowing grass.
"Activity" means the rate of disintegration or transformation or decay of radioactive material. The units of activity are the becquerel (Bq) and the curie (Ci).
"Acute" means a single radiation dose or chemical exposure event or multiple radiation dose or chemical exposure events occurring within a short time (24 hours or less).
"Address of use" means the building or buildings that are identified on the license and where radioactive material may be produced, prepared, received, used, or stored.
"Adult" means an individual 18 or more years of age.
"Agency" means the Radiological Health Program of the Virginia Department of Health.
"Aggregated" means accessible by the breach of a single physical barrier that would allow access to radioactive material in any form, including any devices that contain the radioactive material, when the total activity equals or exceeds a Category 2 quantity of radioactive material as listed in 12VAC5-481-451.
"Agreement state" means any state with which the NRC or the Atomic Energy Commission has entered into an effective agreement under subsection 274b of the Atomic Energy Act of 1954, as amended (42 USC § 2021(b)).
"Airborne radioactive material" means any radioactive material dispersed in the air in the form of dusts, fumes, particulates, mists, vapors, or gases.
"Airborne radioactivity area" means a room, enclosure, or area in which airborne radioactive materials composed wholly or partly of licensed material exist in concentrations:
1. In excess of the derived air concentrations (DACs) specified in Appendix B to 10 CFR Part 20; or
2. To such a degree that an individual present in the area without respiratory protective equipment could exceed, during the hours an individual is present in a week, an intake of 0.6% of the annual limit on intake (ALI) or 12 DAC hours.
"Air kerma" or "K" means kerma in air (see definition of "kerma").
"Air kerma rate" or "AKR" means the air kerma per unit time.
"Air-purifying respirator" means a respirator with an air-purifying filter, cartridge, or canister that removes specific air contaminants by passing ambient air through the air-purifying element.
"Alert" means events may occur, are in progress, or have occurred that could lead to a release of radioactive material but that the release is not expected to require a response by offsite response organizations to protect persons off site.
"Aluminum equivalent" means the thickness of type 1100 aluminum alloy affording the same attenuation, under specified conditions, as the material in question. The nominal chemical composition of type 100 aluminum is 99.00% minimum aluminum, 0.12% copper.
"Analytical x-ray equipment" means equipment used for x-ray diffraction or fluorescence analysis.
"Analytical x-ray system" means a group of components utilizing x-rays or gamma-rays to determine the elemental composition or to examine the microstructure of materials.
"Annual limit on intake" or "ALI" means the derived limit for the amount of radioactive material taken into the body of an adult worker by inhalation or ingestion in a year. ALI is the smaller value of intake of a given radionuclide in a year by the reference man that would result in a committed effective dose equivalent of 0.05 Sv (5 rem) or a committed dose equivalent of 0.5 Sv (50 rem) to any individual organ or tissue. ALI values for intake by ingestion and by inhalation of selected radionuclides are given in Tables 1 and 2 in Appendix B to 10 CFR Part 20.
"Annual refresher safety training" means a review conducted or provided by the licensee or registrant for its employees on radiation safety aspects of industrial radiography. The review shall include, as a minimum, any results of internal inspections, new procedures or equipment, new or revised regulations, and accidents or errors that have been observed. The review shall also provide opportunities for employees to ask safety questions.
"Annually" means at intervals not to exceed one year.
"ANSI" means the American National Standards Institute.
"Approved individual" means an individual whom the licensee has determined to be trustworthy and reliable for unescorted access in accordance with 12VAC5-481-451 and has completed the training required in 12VAC5-481-451.
"Area of use" means a portion of a physical structure that has been set aside for the purpose of producing, preparing, receiving, using, or storing radioactive material.
"Articulated joint" means a joint between two separate sections of a tabletop that provides the capacity for one of the sections to pivot on the line segment along which the sections join.
"As low as is reasonably achievable" or "ALARA" means making every reasonable effort to maintain exposures to radiation as far below the dose limits in these regulations as is practical, consistent with the purpose for which the licensed or registered activity is undertaken, taking into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, and other societal and socioeconomic considerations, and in relation to utilization of nuclear energy and licensed or registered sources of radiation in the public interest.
"Assembler" means any person engaged in the business of assembling, replacing, or installing one or more components into an x-ray system or subsystem. The term includes the owner of an x-ray system or his employee or agent who assembles components into an x-ray system that is subsequently used to provide professional or commercial services.
"Assigned protection factor" or "APF" means the expected workplace level of respiratory protection that would be provided by a properly functioning respirator or a class of respirators to properly fitted and trained users. Operationally, the inhaled concentration can be estimated by dividing the ambient airborne concentration by the APF.
"Associated equipment" means equipment that is used in conjunction with a radiographic exposure device to make radiographic exposures that drive, guide, or come in contact with the source.
"Atmosphere-supplying respirator" means a respirator that supplies the respirator user with breathing air from a source independent of the ambient atmosphere, and includes supplied-air respirators (SARs) and self-contained breathing apparatus (SCBA) units.
"Attenuation block" means a block or stack, having dimensions 20 centimeters by 20 centimeters by 3.8 centimeters, of type 1100 aluminum alloy or other materials having equivalent attenuation. The nominal chemical composition of type 100 aluminum is 99.00% minimum aluminum, 0.12% copper.
"Authorized medical physicist" means an individual who:
1. Meets the requirements in 12VAC5-481-1760 and 12VAC5-481-1790; or
2. Is identified as an authorized medical physicist or teletherapy physicist on:
a. A specific medical use license issued by the NRC or another agreement state;
b. A medical use permit issued by an NRC master material licensee;
c. A permit issued by an NRC or another agreement state broad scope medical use licensee; or
d. A permit issued by an NRC master material license broad scope medical use permittee.
"Authorized nuclear pharmacist" means a pharmacist who:
1. Meets the requirements in 12VAC5-481-1770 and 12VAC5-481-1790;
2. Is identified as an authorized nuclear pharmacist on:
a. A specific license issued by the NRC or another agreement state that authorizes medical use or the practice of nuclear pharmacy;
b. A permit issued by an NRC master material licensee that authorizes medical use or the practice of nuclear pharmacy;
c. A permit issued by an NRC or another agreement state broad scope medical use licensee that authorizes medical use or the practice of nuclear pharmacy; or
d. A permit issued by an NRC master material license broad scope medical use permittee that authorizes medical use or the practice of nuclear pharmacy;
3. Is identified as an authorized nuclear pharmacist by a commercial nuclear pharmacy that has been authorized to identify authorized nuclear pharmacists; or
4. Is designated as an authorized nuclear pharmacist in accordance with 12VAC5-481-440 I 2.
"Authorized user" means a practitioner of the healing arts who:
1. Meets the requirements in 12VAC5-481-1790 and any of the following:
a. 12VAC5-481-1910;
b. 12VAC5-481-1940;
c. 12VAC5-481-1980;
d. 12VAC5-481-1990;
e. 12VAC5-481-2000;
f. 12VAC5-481-2018;
g. 12VAC5-481-2030;
h. 12VAC5-481-2040 A; or
2. Is identified as an authorized user on:
a. A specific license issued by the NRC or another agreement state that authorizes medical use;
b. A permit issued by an NRC master material licensee that authorizes medical use;
c. A permit issued by an NRC or another agreement state broad scope medical use licensee that authorizes medical use; or
d. A permit issued by an NRC master material license broad scope medical use permittee that authorizes medical use.
"Automatic exposure control" or "AEC" means a device that automatically controls one or more technique factors in order to obtain, at a preselected location, a required quantity of radiation (includes devices such as phototimers and ion chambers).
"Background investigation" means the investigation conducted by a licensee or applicant to support the determination of trustworthiness and reliability.
"Background radiation" means radiation from cosmic sources, naturally occurring radioactive materials, that have not been technologically enhanced, including radon, except as a decay product of source or special nuclear material, and including global fallout as it exists in the environment from the testing of nuclear explosive devices, or from past nuclear accidents such as Chernobyl that contribute to background radiation and are not under the control of the licensee or registrant. "Background radiation" does not include sources of radiation from radioactive materials regulated by the agency.
"Barrier" (See "Protective barrier").
"Beam axis" means a line from the source through the centers of the x-ray fields.
"Beam-limiting device" means a device that provides a means to restrict the dimensions of the x-ray field or useful beam.
"Beam monitoring system" means a system designed and installed in the radiation head to detect and measure the radiation present in the useful beam.
"Beam scattering foil" means a thin piece of material (usually metallic) placed in the beam to scatter a beam of electrons in order to provide a more uniform electron distribution in the useful beam.
"Becquerel" or "Bq" means the SI unit of activity. One becquerel is equal to one disintegration or transformation per second (dps or tps).
"Beneficial attribute" means, as used in Part XVI (12VAC5-481-3460 et seq.) of this chapter, the radioactivity of the product necessary to the use of the product.
"Beneficial to the product" (See "Beneficial attribute").
"Bent beam linear accelerator" means a linear accelerator geometry in which the accelerated electron beam must change direction by passing through a bending magnet.
"Bioassay" means the determination of kinds, quantities or concentrations, and, in some cases, the locations of radioactive material in the human body, whether by direct measurement, in-vivo counting, or by analysis and evaluation of materials excreted or removed from the human body. For purposes of these regulations, "radiobioassay" is an equivalent term.
"Board" means the State Board of Health.
"Brachytherapy" means a method of radiation therapy in which sealed sources are utilized to deliver a radiation dose at a distance of up to a few centimeters, by surface, intracavitary, or interstitial application.
"Buffer zone" means a portion of the disposal site that is controlled by the licensee and that lies under the disposal units and between the disposal units and the boundary of the site.
"Byproduct material" means:
1. Any radioactive material (except special nuclear material) yielded in, or made radioactive by, exposure to the radiation incident to the process of producing or using special nuclear material;
2. The tailings or wastes produced by the extraction or concentration of uranium or thorium from ore processed primarily for its source material content, including discrete surface wastes resulting from uranium solution extraction processes. Underground ore bodies depleted by these solution extraction operations do not constitute "byproduct material" within this definition;
3. a. Any discrete source of radium-226 that is produced, extracted, or converted after extraction, before, on, or after August 8, 2005, for use for a commercial, medical, or research activity; or
b. Any material that:
(1) Has been made radioactive by use of a particle accelerator; and
(2) Is produced, extracted, or converted after extraction, before, on, or after August 8, 2005, for use for a commercial, medical, or research activity; and
4. Any discrete source of naturally occurring radioactive material, other than source material, that:
a. The NRC, in consultation with the Administrator of the U.S. Environmental Protection Agency, the U.S. Secretary of Energy, the U.S. Secretary of Homeland Security, and the head of any other appropriate federal agency, determines would pose a threat similar to the threat posed by a discrete source of radium-226 to the public health and safety or the common defense and security; and
b. Before, on, or after August 8, 2005, is extracted or converted after extraction for use in a commercial, medical, or research activity.
"C-arm fluoroscope" means an x-ray system in which the image receptor and x-ray tube housing assembly are connected by a common mechanical support system in order to maintain a desired spatial relationship. This system is designed to allow a change in the projection of the beam through the patient without a change in the position of the patient.
"Cabinet radiography" means industrial radiography conducted in an enclosure or cabinet so shielded that every location on the exterior meets the dose limits for individual members of the public as specified in 12VAC5-481-720.
"Cabinet x-ray system" means an x-ray system with the x-ray tube installed in an enclosure independent of existing architectural structures except the floor on which it may be placed. The cabinet x-ray system is intended to contain at least that portion of a material being irradiated, provide radiation attenuation, and exclude personnel from its interior during generation of radiation. Included are all x-ray systems designed primarily for the inspection of carry-on baggage at airline, railroad, and bus terminals, and in similar facilities. An x-ray tube used within a shielded part of a building, or x-ray equipment that may temporarily or occasionally incorporate portable shielding, is not considered a cabinet x-ray system.
"Calendar quarter" means not less than 12 consecutive weeks nor more than 14 consecutive weeks. The first calendar quarter of each year shall begin in January and subsequent calendar quarters shall be so arranged such that no day is included in more than one calendar quarter and no day in any one year is omitted from inclusion within a calendar quarter. The method observed by the licensee or registrant for determining calendar quarters shall only be changed at the beginning of a year.
"Calibration" means the determination of (i) the response or reading of an instrument relative to a series of known radiation values over the range of the instrument or (ii) the strength of a source of radiation relative to a standard.
"Camera" (See "Radiographic exposure device").
"Carrier" means a person engaged in the transportation of passengers or property by land or water as a common, contract, or private carrier, or by civil aircraft.
"Cassette holder" means a device, other than a spot-film device, that supports or fixes the position of an x-ray film (imaging) cassette during an x-ray exposure.
"Category 1 quantities of radioactive material" or "Category 1" means a quantity of radioactive material meeting or exceeding the Category 1 threshold in Table 1 of 12VAC5-481-451. This is determined by calculating the ratio of the total activity of each radionuclide to the Category 1 threshold for that radionuclide and adding the ratios together. If the sum is equal to or exceeds 1, the quantity would be considered a Category 1 quantity. Category 1 quantities of radioactive material do not include the radioactive material contained in any fuel assembly, subassembly, fuel rod, or fuel pellet.
"Category 2 quantities of radioactive material" or "Category 2" means a quantity of radioactive material meeting or exceeding the Category 2 threshold but less than the Category 1 threshold in Table 1 of 12VAC5-481-451. This is determined by calculating the ratio of the total activity of each radionuclide to the Category 2 threshold for that radionuclide and adding the ratios together. If the sum is equal to or exceeds 1, the quantity would be considered a Category 2 quantity. Category 2 quantities of radioactive material do not include the radioactive material contained in any fuel assembly, subassembly, fuel rod, or fuel pellet.
"Certifiable cabinet x-ray system" means an existing uncertified x-ray system that has been modified to meet the certification requirements specified in 21 CFR 1020.40.
"Certificate holder" means a person who has been issued a certificate of compliance or other package approval by the NRC.
"Certificate of compliance" or "CoC" means the certificate issued by the NRC that approves the design of a package for the transportation of radioactive material.
"Certified cabinet x-ray system" means an x-ray system that has been certified in accordance with 21 CFR 1010.2 as being manufactured and assembled pursuant to the provisions of 21 CFR 1020.40.
"Certified components" means components of x-ray systems that are subject to regulations promulgated under P.L. 90-602, the Radiation Control for Health and Safety Act of 1968 of the Food and Drug Administration.
"Certifying entity" means an independent certifying organization meeting the agency's requirements for documenting applicant's training in topics set forth in 12VAC5-481-1320 or equivalent state or NRC regulations.
"CFR" means Code of Federal Regulations.
"Chelating agent" means amine polycarboxylic acids, hydroxycarboxylic acids, gluconic acid, and polycarboxylic acids.
"Chemical description" means a description of the principal chemical characteristics of a low-level radioactive waste.
"Class" means a classification scheme for inhaled material according to its rate of clearance from the pulmonary region of the lung. Materials are classified as D, W, or Y, which applies to a range of clearance half-times: for Class D, Days, of less than 10 days; for Class W, Weeks, from 10 to 100 days; and for Class Y, Years, of greater than 100 days. For purposes of these regulations, "lung class" and "inhalation class" are equivalent terms.
"Closed transport vehicle" means a transport vehicle equipped with a securely attached exterior enclosure that during normal transportation restricts the access of unauthorized persons to the cargo space containing the radioactive material. The enclosure may be either temporary or permanent but shall limit access from top, sides, and ends. In the case of packaged materials, it may be of the "see-through" type.
"cm" means centimeters.
"Coefficient of variation or "C" means the ratio of the standard deviation to the mean value of a population of observations. It is estimated using the following equation:

where:
s = Standard deviation of the observed values;
X¯ = Mean value of observations in sample;
xi = ith observation in sample;
n = Number of observations in sample.
"Collective dose" means the sum of the individual doses received in a given period of time by a specified population from exposure to a specified source of radiation.
"Collimator" means a device used to limit the size, shape, and direction of the primary radiation beam. For industrial radiography it means a radiation shield that is placed on the end of the guide tube or directly onto a radiographic exposure device to restrict the size of the radiation beam when the sealed source is cranked into position to make a radiographic exposure.
"Commencement of construction" means taking any action defined as "construction" or any other activity at the site of a facility subject to the regulations in this chapter that has a reasonable nexus to radiological health and safety.
"Committed dose equivalent" or "HT,50" means the dose equivalent to organs or tissues of reference (T) that will be received from an intake of radioactive material by an individual during the 50-year period following the intake.
"Committed effective dose equivalent" or "HE,50" means the sum of the products of the weighting factors (wT) applicable to each of the body organs or tissues that are irradiated and the committed dose equivalent to each of these organs or tissues (HE,50 = S (wT HT,50)).
"Computed tomography" means the production of a tomogram by the acquisition and computer processing of x-ray transmission data.
"Computed tomography dose index" means the integral from -7T to +7T of the dose profile along a line perpendicular to the tomographic plane divided by the product of the nominal tomographic section thickness and the number of tomograms produced in a single scan, that is:

where:
z = Position along a line perpendicular to the tomographic plane;
D(z) = Dose at position z;
T = Nominal tomographic section thickness;
n = Number of tomograms produced in a single scan.
This definition assumes that the dose profile is centered around z = 0 and that, for a multiple tomogram system, the scan increment between adjacent scans is nT.
"Computer-readable medium" means that the regulatory agency's computer can transfer the information from the medium into its memory.
"Consignee" means the designated receiver of the shipment of low-level radioactive waste.
"Consignment" means each shipment of a package or groups of packages or load of radioactive material offered by a shipper for transport.
"Consortium" means an association of medical use licensees and a PET radionuclide production facility in the same geographical area that jointly own or share in the operation and maintenance cost of the PET radionuclide production facility that produces PET radionuclides for use in producing radioactive drugs within the consortium for noncommercial distributions among its associated members for medical use. The PET radionuclide production facility within the consortium must be located at an educational institution or a federal facility or a medical facility.
"Constraint" means each shipment of a package or groups of packages or load of radioactive material offered by a shipper for transport.
"Constraint" or "dose constraint" means a value above which specified licensee actions are required.
"Construction" means the installation of foundations, or in-place assembly, erection, fabrication, or testing for any structure, system, or component of a facility or activity subject to this chapter. The term "construction" does not include:
1. Changes for temporary use of the land for public recreational purposes;
2. Site exploration, including necessary borings to determine foundation conditions or other preconstruction monitoring to establish background information related to the suitability of the site, the environmental impacts of construction or operation, or the protection of environmental values;
3. Preparation of the site for construction of the facility, including clearing of the site, grading, installation of drainage, erosion and other environmental mitigation measures, and construction of temporary roads and borrow areas;
4. Erection of fences and other access control measures that are not related to the safe use of, or security of, radiological materials subject to this chapter;
5. Excavation;
6. Erection of support buildings (e.g., construction equipment storage sheds, warehouse and shop facilities, utilities, concrete mixing plants, docking and unloading facilities, and office buildings) for use in connection with the construction of the facility;
7. Building of service facilities (e.g., paved roads, parking lots, railroad spurs, exterior utility and lighting systems, potable water systems, sanitary sewerage treatment facilities, and transmission lines);
8. Procurement or fabrication of components or portions of the proposed facility occurring at other than the final, in-place location at the facility; or
9. Taking any other action that has no reasonable nexus to radiological health and safety.
"Contact therapy system" means a therapeutic radiation machine with a short target to skin distance (TSD), usually less than five centimeters.
"Contamination" means, as applicable to Part XIII (12VAC5-481-2950 et seq.) of this chapter, the presence of a radioactive substance on a surface in quantities in excess of 0.4 Bq/cm2 (1 x 10-5 µCi/cm2) for beta and gamma emitters and low toxicity alpha emitters, or 0.04 Bq/cm2 (1 x 10-6 µCi/cm2) for all other alpha emitters.
1. Fixed contamination means contamination that cannot be removed from a surface during normal conditions of transport.
2. Nonfixed contamination means contamination that can be removed from a surface during normal conditions of transport.
"Contrast scale" means the change in the linear attenuation coefficient per CTN relative to water, that is:

where:
µx = Linear attenuation coefficient of the material of interest;
µw = Linear attenuation coefficient of water;
 = of the material of interest;
= of the material of interest;
 = of water.
= of water.
"Control cable" or "drive" means the cable that is connected to the source assembly and used to drive the source to and from the exposure location.
"Control drive mechanism" means a device that enables the source assembly to be moved into and out of the exposure device.
"Control panel" means that part of the x-ray control upon which are mounted the switches, knobs, pushbuttons, and other hardware necessary for manually setting the technique factors.
"Control tube" means a protective sheath for guiding the control cable. The control tube connects the control drive mechanism to the radiographic exposure device.
"Controlled area" means an area, outside of a restricted area but inside the site boundary, access to which can be limited by the licensee for any reason.
"Conventional simulator" means any x-ray system designed to reproduce the geometric conditions of the radiation therapy equipment.
"Conveyance" means:
1. For transport by public highway or rail any transport vehicle or large freight container;
2. For transport by water any vessel, or any hold, compartment, or defined deck area of a vessel including any transport vehicle on board the vessel; and
3. For transport by any aircraft.
"Cooling curve" means the graphical relationship between heat units stored and cooling time.
"Cradle" means either:
1. A removable device that supports and may restrain a patient above an x-ray table; or
2. A device:
a. Whose patient support structure is interposed between the patient and the image receptor during normal use;
b. Which is equipped with means for patient restraint; and
c. Which is capable of rotation about its long (longitudinal) axis.
"Critical group" means the group of individuals reasonably expected to receive the greatest exposure to residual radioactivity for any applicable set of circumstances.
"Criticality safety index" or "CSI" means the dimensionless number (rounded up to the next tenth) assigned to and placed on the label of a fissile material package, to designate the degree of control of accumulation of packages, overpacks or freight containers containing fissile material during transportation. Determination of the criticality safety index is described in Part XIII (12VAC5-481-2950 et seq.) 12VAC5-481-3040, 12VAC5-481-3051, and 10 CFR 71.59. The criticality safety index for an overpack, freight container, consignment, or conveyance containing fissile material packages is the arithmetic sum of the critically safety indices of all the fissile material packages contained within the overpack, freight container, consignment, or conveyance.
"CS" (See "Contrast scale").
"CT" (See "Computed tomography").
"CT conditions of operation" means all selectable parameters governing the operation of a CT x-ray system including, but not limited to, nominal tomographic section thickness, filtration, and the technique factors as defined in these regulations.
"CTDI" (See "Computed tomography dose index").
"CT gantry" means the tube housing assemblies, beam-limiting devices, detectors, and the supporting structures and frames which hold these components.
"CTN" (See "CT number").
"CT number" means the number used to represent the x-ray attenuation associated with each elemental area of the CT image.

where:
k = A constant, a normal value of 1,000 when the Hounsfield scale of CTN is used;
µx = Linear attenuation coefficient of the material of interest;
µw = Linear attenuation coefficient of water.
"Cumulative air kerma" means the total air kerma accrued from the beginning of an examination or procedure and includes all contribution from fluoroscopic and radiographic irradiation.
"Curie" is a unit of quantity of activity. One curie (Ci) is that quantity of radioactive material that decays at the rate of 3.7E+10 disintegrations or transformations per second (dps or tps).
"Custodial agency" means an agency of the government designated to act on behalf of the government owner of the disposal site.
"Declared pregnant woman" means a woman who has voluntarily informed the licensee, in writing, of her pregnancy and the estimated date of conception. The declaration remains in effect until the declared pregnant woman withdraws the declaration in writing or is no longer pregnant.
"Decommission" means to remove a facility or site safely from service and reduce residual radioactivity to a level that permits release of the property for unrestricted use and termination of the license or release of the property under restricted conditions and termination of the license.
"Decontamination facility" means a facility operating under a commission or agreement state license whose principal purpose is decontamination of equipment or materials to accomplish recycle, reuse, or other waste management objectives, and, for purposes of this chapter, is not considered to be a consignee for LLW shipments.
"Dedicated check source" means a radioactive source that is used to assure the constant operation of a radiation detection or measurement device over several months or years. This source may also be used for other purposes.
"Deep dose equivalent" or "Hd," which applies to external whole body exposure, means the dose equivalent at a tissue depth of one centimeter (1000 mg/cm²).
"Demand respirator" means an atmosphere-supplying respirator that admits breathing air to the facepiece only when a negative pressure is created inside the facepiece by inhalation.
"Department of Energy" means the Department of Energy established by P.L. 95-91, August 4, 1977, 91 Stat. 565, 42 USC § 7101 et seq., to the extent that the Department exercises functions formerly vested in the Atomic Energy Commission, its Chairman, members, officers and components and transferred to the Energy Research and Development Administration and to the Administrator thereof pursuant to §§ 104(b), (c) and (d) of the Energy Reorganization Act of 1974 (P.L. 93-438, October 11, 1974, 88 Stat. 1233 at 1237, 42 USC § 5814, effective January 19, 1975) and retransferred to the U.S. Secretary of Energy pursuant to § 301(a) of the Department of Energy Organization Act (P.L. 95-91, August 4, 1977, 91 Stat. 565 at 577-578, 42 USC § 7151, effective October 1, 1977).
"Depleted uranium" means the source material uranium in which the isotope uranium-235 is less than 0.711 weight percentage of the total uranium present. Depleted uranium does not include special nuclear material.
"Derived air concentration" or "DAC" means the concentration of a given radionuclide in air which, if breathed by the reference man for a working year of 2,000 hours under conditions of light work, results in an intake of one ALI. For purposes of these regulations, the condition of light work is an inhalation rate of 1.2 cubic meters of air per hour for 2,000 hours in a year. DAC values are given in Appendix B to 10 CFR Part 20.
"Derived air concentration-hour" or "DAC hour" means the product of the concentration of radioactive material in air, expressed as a fraction or multiple of the derived air concentration for each radionuclide, and the time of exposure to that radionuclide, in hours. A licensee or registrant may take 2,000 DAC hours to represent one ALI, equivalent to a committed effective dose equivalent of 0.05 Sv (5 rem).
"Detector" (See "Radiation detector").
"Deuterium" means, for the purposes of Part XIII (12VAC5-481-2950 et seq.) of this chapter, deuterium and any deuterium compounds, including heavy water, in which the ratio of deuterium atoms to hydrogen atoms exceeds 1:5000.
"Diagnostic clinical procedures manual" means a collection of written procedures that describes each method (and other instructions and precautions) by which the licensee performs diagnostic clinical procedures, where each diagnostic clinical procedure has been approved by the authorized user and includes the radiopharmaceutical, dosage, and route of administration.
"Diagnostic source assembly" means the tube housing assembly with a beam-limiting device attached.
"Diagnostic x-ray system" means an x-ray system designed for irradiation of any part of the human or animal body for the purpose of diagnosis or visualization.
"Direct scattered radiation" means that scattered radiation that has been deviated in direction only by materials irradiated by the useful beam (See "Scattered radiation").
"Discrete source" means a radionuclide that has been processed so that its concentration within a material has been purposely increased for use for commercial, medical, or research activities.
"Disposable respirator" means a respirator for which maintenance is not intended and that is designed to be discarded after excessive breathing resistance, sorbent exhaustion, physical damage, or end-of-service-life renders it unsuitable for use. Examples of this type of respirator are a disposable half-mask respirator and a disposable escape-only self-contained breathing apparatus (SCBA).
"Disposal" means the isolation of wastes from the biosphere inhabited by man and his food chains by emplacement in a land disposal facility.
"Disposal container" means a container principally used to confine low-level radioactive waste during disposal operations at a land disposal facility (also see "high integrity container"). Note that for some shipments, the disposal container may be the transport package.
"Disposal site" means that portion of a land disposal facility that is used for disposal of waste. It consists of disposal units and a buffer zone.
"Disposal unit" means a discrete portion of the disposal site into which waste is placed for disposal. For near-surface disposal, the unit is usually a trench.
"Distinguishable from background" means that the detectable concentration of a radionuclide is statistically different from the background concentration of that radionuclide in the vicinity of the site or, in the case of structures, in similar materials using adequate measurement technology, survey, and statistical techniques.
"Diversion" means the unauthorized movement of radioactive material subject to 12VAC5-481-451 to a location different from the material's authorized destination inside or outside of the site at which the material is used or stored.
"Dose" is a generic term that means absorbed dose, dose equivalent, effective dose equivalent, committed dose equivalent, committed effective dose equivalent, total organ dose equivalent, or total effective dose equivalent. For purposes of these regulations, "radiation dose" is an equivalent term.
"Dose commitment" means the total radiation dose to a part of the body that will result from retention in the body of radioactive material. For purposes of estimating the dose commitment, it is assumed that from the time of intake the period of exposure to retained material will not exceed 50 years.
"Dose equivalent" or "HT" means the product of the absorbed dose in tissue, quality factor, and all other necessary modifying factors at the location of interest. The units of dose equivalent are the sievert (Sv) and rem.
"Dose limits" means the permissible upper bounds of radiation doses established in accordance with these regulations. For purposes of these regulations, "limits" is an equivalent term.
"Dose monitor unit" or "DMU" means a unit response from the beam monitoring system from which the absorbed dose can be calculated.
"Dose profile" means the dose as a function of position along a line.
"Dosimetry processor" means an individual or an organization that processes and evaluates individual monitoring devices in order to determine the radiation dose delivered to the monitoring devices.
"Doubly encapsulated sealed source" means a sealed source in which the radioactive material is sealed within an inner capsule and that capsule is sealed within an outer capsule.
"Drive cable" (See "Control cable").
"Effective dose equivalent" or "HE" means the sum of the products of the dose equivalent (HT) to each organ or tissue and the weighting factor (wT) applicable to each of the body organs or tissues that are irradiated (HE = S wTHT).
"Electronic brachytherapy" means a method of radiation therapy where an electrically generated source of ionizing radiation is placed in or near the tumor or target tissue to deliver therapeutic radiation dosage.
"Electronic brachytherapy device" means the system used to produce and deliver therapeutic radiation including the x-ray tube, the control mechanism, the cooling system, and the power source.
"Electronic brachytherapy source" means the x-ray tube component used in an electronic brachytherapy device.
"Elemental area" means the smallest area within a tomogram for which the x-ray attenuation properties of a body are depicted. (See also "Picture element").
"Embryo/fetus" means the developing human organism from conception until the time of birth.
"Energy compensation source" or "ECS" means a small sealed source, with an activity not exceeding 3.7 MBq (100 µCi), used within a logging tool, or other tool components, to provide a reference standard to maintain the tool's calibration when in use.
"Engineered barrier" means a manmade structure or device that is intended to improve the land disposal facility's ability to meet the performance objectives in these regulations.
"Enriched uranium" (See "Uranium - natural, depleted, enriched").
"Entrance or access point" means any opening through which an individual or extremity of an individual could gain access to radiation areas or to licensed or registered radioactive materials. This includes entry or exit portals of sufficient size to permit human entry, irrespective of their intended use.
"EPA identification number" means the number received by a transporter following application to the Administrator of the U.S. Environmental Protection Agency as required by 40 CFR Part 263.
"Equipment" (See "x-ray equipment").
"Escorted access" means accompaniment while in a security zone by an approved individual who maintains continuous direct visual surveillance at all times over an individual who is not approved for unescorted access.
"Exclusive use" means the sole use by a single consignor of a conveyance for which all initial, intermediate, and final loading and unloading are carried out in accordance with the direction of the consignor or consignee. The consignor and the carrier must ensure that any loading or unloading is performed by personnel having radiological training and resources appropriate for safe handling of the consignment. The consignor must issue specific instructions, in writing, for maintenance of exclusive use shipment controls, and include them with the shipping paper information provided to the carrier by the consignor.
"Explosive material" means any chemical compound, mixture, or device that produces a substantial instantaneous release of gas and heat spontaneously or by contact with sparks or flame.
"Exposure" means being exposed to ionizing radiation or to radioactive material.
"Exposure head" means a device that locates the gamma radiography sealed source in the selected working position.
"Exposure rate" means the exposure per unit of time, such as roentgen per minute and milliroentgen per hour.
"External beam radiation therapy" means therapeutic irradiation in which the source of radiation is at a distance from the body.
"External dose" means that portion of the dose equivalent received from any source of radiation outside the body.
"Extremity" means hand, elbow, arm below the elbow, foot, knee, and leg below the knee.
"Facility" means the location, building, vehicle, or complex under one administrative control, at which one or more radiation machines are installed, located or used.
"Fail-safe characteristics" means a design feature that causes beam port shutters to close, or otherwise prevents emergence of the primary beam, upon the failure of a safety or warning device.
"Field emission equipment" means equipment that uses an x-ray tube in which electron emission from the cathode is due solely to the action of an electric field.
"Field-flattening filter" means a filter used to homogenize the absorbed dose rate over the radiation field.
"Field station" means a facility where radioactive sources may be stored or used and from which equipment is dispatched to temporary jobsites.
"Filter" means material placed in the useful beam to preferentially absorb selected radiations. It also means material placed in the useful beam to change beam quality in therapeutic radiation machines subject to Part XV (12VAC5-481-3380 et seq.) of this chapter.
"Filtering facepiece" or "dusk mask" means a negative pressure particulate respirator with a filter as an integral part of the facepiece or with the entire facepiece composed of the filtering medium, not equipped with elastomeric sealing surfaces and adjustable straps.
"Fingerprint orders" means the requirements of 12VAC5-481-451 B or orders issued by the U.S. Nuclear Regulatory Commission or the legally binding requirements issued by agreement states that require fingerprints and criminal history records checks for individuals with unescorted access to Category 1 and Category 2 quantities of radioactive material or safeguards information-modified handling.
"Fissile material" means the radionuclides uranium-233, uranium-235, plutonium-239, and plutonium-241, or any combination of these radionuclides. "Fissile material" means the fissile nuclides themselves, not material containing fissile nuclides. Unirradiated natural uranium and depleted uranium and natural uranium or depleted uranium, that has been irradiated in thermal reactors only, are not included in this definition. Certain exclusions from fissile material controls are provided in 10 CFR 71.15.
1. Fissile Class I: A package that may be transported in unlimited numbers and in any arrangement, and that requires no nuclear criticality safety controls during transportation. A transport index is not assigned for purposes of nuclear criticality safety but may be required because of external radiation levels.
2. Fissile Class II: A package that may be transported together with other packages in any arrangement but, for criticality control, in numbers that do not exceed an aggregate transport index of 50. These shipments require no other nuclear criticality safety control during transportation. Individual packages may have a transport index not less than 0.1 and not more than 10.
"Fissile material package" means a fissile material packaging together with its fissile material contents.
"Fit factor" means a quantitative estimate of the fit of a particular respirator to a specific individual, and typically estimates the ratio of the concentration of a substance in ambient air to its concentration inside the respirator when worn.
"Fit test" means the use of a protocol to qualitatively or quantitatively evaluate the fit of a respirator on an individual.
"Fluoroscopic imaging assembly" means a subsystem in which x-ray photons produce a set of fluoroscopic images or radiographic images recorded from the fluoroscopic image receptor. It includes the image receptors, electrical interlocks, if any, and structural material providing linkage between the image receptor and diagnostic source assembly.
"Fluoroscopic irradiation time" means the cumulative duration during an examination or procedure of operator-applied continuous pressure to the device, enabling x-ray tube activation in any fluoroscopic mode of operation.
"Fluoroscopy" means a technique for generating x-ray images and presenting them simultaneously and continuously as visible images. This term has the same meaning as the term "radioscopy" in the standards of the International Electrotechnical Commission.
"Focal spot" or "actual" means the area projected on the anode of the x-ray tube bombarded by the electrons accelerated from the cathode and from which the useful beam originates.
"Former Atomic Energy Commission or NRC licensed facilities" means nuclear reactors, nuclear fuel reprocessing plants, uranium enrichment plants, or critical mass experimental facilities where Atomic Energy Commission or NRC licenses have been terminated.
"Gantry" means that part of a radiation therapy system supporting and allowing movements of the radiation head about a center of rotation.
"Generally applicable environmental radiation standards" means standards issued by the U.S. Environmental Protection Agency under the authority of the Atomic Energy Act of 1954, as amended, (42 USC § 2011 et seq.) that impose limits on radiation exposures or levels, or concentrations or quantities of radioactive material, in the general environment outside the boundaries of locations under the control of persons possessing or using radioactive material.
"General environment" means, as used in Part XVI (12VAC5-481-3460 et seq.) of this chapter, the total terrestrial, atmospheric, and aquatic environments outside the site boundary within which any activity, operation, or process authorized by a general or specific license issued under Part XVI, is performed.
"General purpose radiographic x-ray system" means any radiographic x-ray system that, by design, is not limited to radiographic examination of specific anatomical regions.
"Generator" means a licensee who (i) is a waste generator as defined in this chapter, or (ii) is the licensee to whom waste can be attributed within the context of the Low-Level Radioactive Waste Policy Amendments Act of 1985 (42 USC § 2021) (e.g., waste generated as a result of decontamination or recycle activities).
"Gonad shield" means a protective barrier for the testes or ovaries.
"Gray" or "Gy" means the SI unit of absorbed dose. One gray is equal to an absorbed dose of one joule per kilogram (100 rad).
"Guide tube (protection sheath)" means a flexible or rigid tube, or "J" tube, for guiding the source assembly and the attached control cable from the exposure device to the exposure head. The guide tube may also include the connections necessary for attachment to the exposure device and to the exposure head.
"Half-value layer" or "HVL" means the thickness of a specified material that attenuates the beam of radiation to an extent that the AKR is reduced by one-half of its original value. In this definition, the contribution of all scattered radiation, other than any which might be present initially in the beam concerned, is deemed to be excluded.
"Hand-held radiographic unit" means x-ray equipment that is designed to be hand-held during operation.
"Hands-on experience" means experience in all of those areas considered to be directly involved in the radiography process, and includes taking radiographs, calibration of survey instruments, operational and performance testing of survey instruments and devices, film development, posting of radiation areas, transportation of radiography equipment, posting of records and radiation area surveillance, etc., as applicable. Excessive time spent in only one or two of these areas, such as film development or radiation area surveillance, should not be counted toward the 2,000 hours of hands-on experience required for a radiation safety officer in 12VAC5-481-1310 B 2 or the hands-on experience for a radiographer as required by 12VAC5-481-1320 A.
"Hazardous waste" means those wastes designated as hazardous by the U.S. Environmental Protection Agency regulations in 40 CFR Part 261.
"Healing arts" means the art or science or group of arts or sciences dealing with the prevention and cure or alleviation of ailments, diseases or infirmities, and has the same meaning as "medicine" when the latter term is used in its comprehensive sense.
"Healing arts screening" means the testing of human beings using x-ray machines for the detection or evaluation of health indications when such tests are not specifically and individually ordered by a licensed practitioner of the healing arts legally authorized to prescribe such x-ray tests for the purpose of diagnosis or treatment.
"Heat unit" means a unit of energy equal to the product of the peak kilovoltage, milliamperes, and seconds, such as (kVp) times (mA) times (seconds).
"Helmet" means a rigid respiratory inlet covering that also provides head protection against impact and penetration.
"High integrity container" or "HIC" means a container commonly designed to meet the structural stability requirements of 12VAC5-481-2572 and to meet U.S. Department of Transportation requirements for a Type A package.
"High radiation area" means an area, accessible to individuals, in which radiation levels from radiation sources external to the body could result in an individual receiving a dose equivalent in excess of one mSv (0.1 rem) in one hour at 30 centimeters from any source of radiation or 30 centimeters from any surface that the radiation penetrates.
"Hood" means a respiratory inlet covering that completely covers the head and neck and may also cover portions of the shoulders and torso.
"Human use" means the internal or external administration of radiation or radioactive material to human beings.
"Hydrogeologic unit" means any soil or rock unit or zone which by virtue of its porosity or permeability, or lack thereof, has a distinct influence on the storage or movement of groundwater.
"Image intensifier" means a device, installed in its housing, that instantaneously converts an x-ray pattern into a corresponding light image of higher intensity.
"Image receptor" means any device, such as a fluorescent screen, radiographic film, x-ray image intensifier tube, solid-state detector, or gaseous detector that transforms incident x-ray photons either into a visible image or into another form that can be made into a visible image by further transformations. In those cases where means are provided to preselect a portion of the image receptor, the term "image receptor" shall mean the preselected portion of the device.
"Image receptor support device" means, for mammographic systems, that part of the system designed to support the image receptor during mammographic examination and to provide a primary protective barrier.
"Inadvertent intruder" means a person who might occupy the disposal site after closure and engage in normal activities, such as agriculture, dwelling construction, or other pursuits in which an individual might be unknowingly exposed to radiation from the waste.
"Indian tribe" means an Indian or Alaska Native tribe, band, nation, pueblo, village, or community that the U.S. Secretary of the Interior acknowledges to exist as an Indian tribe pursuant to the Federally Recognized Indian Tribe List Act of 1994 (25 USC § 479a).
"Independent certifying organization" means an independent organization that meets the agency's criteria for documenting applicant's training in topics set forth in 12VAC5-481-1320 or equivalent agreement state or NRC regulations.
"Individual" means any human being.
"Individual monitoring" means the assessment of:
1. Dose equivalent (i) by the use of individual monitoring devices or (ii) by the use of survey data; or
2. Committed effective dose equivalent (i) by bioassay or (ii) by determination of the time-weighted air concentrations to which an individual has been exposed, that is, DAC hours. (See the definition of DAC).
"Individual monitoring devices" means devices designed to be worn by a single individual for the assessment of dose equivalent. For purposes of these regulations, "personnel dosimeter" and "dosimeter" are equivalent terms. Examples of individual monitoring devices are film badges, thermoluminescent dosimeters (TLDs), pocket ionization chambers, optically stimulated luminescence (OSL) dosimeters and personal air sampling devices.
"Industrial radiography" means an examination of the structure of materials by the nondestructive method of utilizing ionizing radiation to make radiographic images.
"Inhalation class" (See "Class").
"Injection tool" means a device used for controlled subsurface injection of radioactive tracer material.
"Inspection" means an official examination or observation including, but not limited to, tests, surveys, and monitoring to determine compliance with rules, regulations, orders, requirements, and conditions of the agency.
"Institutional controls" means: (i) permanent markers placed at a disposal site, (ii) public records and archives, (iii) government ownership and regulations regarding land or resource use, and (iv) other methods of preserving knowledge about the location, design, and contents of a disposal system.
"Instrument traceability" (for ionizing radiation measurements) means the ability to show that an instrument has been calibrated at specified time intervals using a national standard or a transfer standard. If a transfer standard is used, the calibration must be at a laboratory accredited by a program that requires continuing participation in measurement quality assurance with the National Institute of Standards and Technology or other equivalent national or international program.
"Intensity modulated radiation therapy" or "IMRT" means radiation therapy that uses nonuniform radiation beam intensities that have been determined by various computer-based optimization techniques.
"Interlock" means a device arranged or connected such that the occurrence of an event or condition is required before a second event or condition can occur or continue to occur.
"Internal dose" means that portion of the dose equivalent received from radioactive material taken into the body.
"Interruption of irradiation" means the stopping of irradiation with the possibility of continuing irradiation without resetting of operating conditions at the control panel.
"Intruder barrier" means a sufficient depth of cover over the waste that inhibits contact with waste and helps to ensure that radiation exposures to an inadvertent intruder will meet the performance objectives set forth in these regulations, or engineered structures that provide equivalent protection to the inadvertent intruder.
"Irradiation" means the exposure of matter to ionizing radiation.
"Irradiator" means a facility that uses radioactive sealed sources for the irradiation of objects or materials and in which radiation dose rates exceeding five grays (500 rads) per hour exist at one meter from the sealed radioactive sources in air or water, as applicable for the irradiator type, but does not include irradiators in which both the sealed source and the area subject to irradiation are contained within a device and are not accessible to personnel.
"Irradiator operator" means an individual who has successfully completed the training and testing described in 12VAC5-481-2830 and is authorized by the terms of the license to operate the irradiator without a supervisor present.
"Irradiator operator supervisor" means an individual who meets the requirements for an irradiator operator and who physically oversees operation of the irradiator by an individual who is currently receiving training and testing described in 12VAC5-481-2830.
"Isocenter" means the center of the smallest sphere through which the beam axis passes when the equipment moves through a full range of rotations about its common center.
"kBq" means kilobecquerel.
"Kerma" or "K" means the quantity defined by the International Commission on Radiation Units and Measurements. The kerma is the quotient of dEtr by dm, where dEtr is the sum of the initial kinetic energies of all charged particles liberated by uncharged particles in a mass dm of materials; thus K=dEtr/dm, in units of J/kg, where the special name for the units of kerma is gray (Gy). When the materials is air, the quantity is referred to as "air kerma."
"Kilovolt" or "kV" means the energy equal to that acquired by a particle with one electron charge in passing through a potential difference of 1,000 volts in a vacuum. Current convention is to use kV for photons and keV for electrons.
"Kilovolts peak" (See "Peak tube potential").
"kV" means kilovolts.
"kVp" (See "Peak tube potential").
"kWs" means kilowatt second.
"Land disposal facility" means the land, buildings, structures and equipment that are intended to be used for the disposal of wastes into the subsurface of the land. For purposes of this chapter, a "geologic repository" as defined in 10 CFR Part 60 or 10 CFR Part 63 is not considered a land disposal facility.
"Last image hold radiograph" or "LIH" means an image obtained either by retaining one or more fluoroscopic images, which may be temporarily integrated, at the end of a fluoroscopic exposure or by initiating a separate and distinct radiographic exposure automatically and immediately in conjunction with termination of the fluoroscopic exposure.
"Lay-barge radiography" means industrial radiography performed on any water vessel used for laying pipe.
"Lead equivalent" means the thickness of the material in question affording the same attenuation, under specified conditions, as lead.
"Leakage radiation" means radiation emanating from the diagnostic source assembly or the radiation therapy system except for:
1. The useful beam; and
2. Radiation produced when the exposure switch or timer is not activated.
"Leakage technique factors" means the technique factors associated with the diagnostic source assembly that are used in measuring leakage radiation. They are defined as follows:
1. For diagnostic source assemblies intended for capacitor energy storage equipment, the maximum-rated peak tube potential and the maximum-rated number of exposures in an hour for operation at the maximum-rated peak tube potential with the quantity of charge per exposure being 10 millicoulombs, (10 mAs), or the minimum obtainable from the unit, whichever is larger;
2. For diagnostic source assemblies intended for field emission equipment rated for pulsed operation, the maximum-rated peak tube potential and the maximum-rated number of x-ray pulses in an hour for operation at the maximum-rated peak tube potential; or
3. For all other diagnostic source assemblies, the maximum-rated peak tube potential and the maximum-rated continuous tube current for the maximum-rated peak tube potential.
"Lens dose equivalent" or "LDE" applies to the external exposure of the lens of the eye and is taken as the dose equivalent at a tissue depth of 0.3 cm (300 mg/cm2).
"License" means a license issued by the agency in accordance with the regulations adopted by the board.
"Licensed material" means radioactive material received, possessed, used, transferred or disposed of under a general or specific license issued by the agency.
"Licensee" means any person who is licensed by the agency in accordance with these regulations and the Act.
"Light field" means the area illuminated by light, simulating the radiation field.
"Limits" (See "Dose limits").
"Line-voltage regulation" means the difference between the no-load and the load line potentials expressed as a percent of the load line potential as follows:
Percent line-voltage regulation = 100 (Vn-Vl)/Vl
where:
Vn = No-load line potential; and
Vl = Load line potential.
"Lixiscope" means a portable light-intensified imaging device using a sealed source.
"Local components" means part of an analytical x-ray system and include areas that are struck by x-rays such as radiation source housings, port and shutter assemblies, collimators, sample holders, cameras, goniometers, detectors, and shielding, but do not include power supplies, transformers, amplifiers, readout devices, and control panels.
"Local law-enforcement agency" or "LLEA" means a public or private organization that has been approved by a federal, state, or local government to carry firearms and make arrests, and is authorized and has the capability to provide an armed response in the jurisdiction where the licensed Category 1 or Category 2 quantity of radioactive material is used, stored, or transported.
"Logging assistant" means any individual who, under the personal supervision of a logging supervisor, handles sealed sources or tracers that are not in logging tools or shipping containers or who performs surveys required by Part XIV (12VAC5-481-3140 et seq.) of this chapter.
"Logging supervisor" means the individual who uses licensed material or provides personal supervision in the use of licensed material at a temporary jobsite and who is responsible to the licensee for assuring compliance with the requirements of this chapter and the conditions of the license.
"Logging tool" means a device used subsurface to perform well-logging.
"Loose-fitting facepiece" means a respiratory inlet covering that is designed to form a partial seal with the face.
"Lost or missing licensed material" means licensed (or registered) source of radiation whose location is unknown. This definition includes, but is not limited to, radioactive material that has been shipped but has not reached its planned destination and whose location cannot be readily traced in the transportation system.
"Lot tolerance percent defective" means, expressed in percent defective, the poorest quality in an individual inspection lot that should be accepted.
"Low specific activity material" or "LSA material" means radioactive material with limited specific activity that is nonfissile or is excepted under 12VAC5-481-2970 C, and that satisfies the descriptions and limits set forth below. Shielding materials surrounding the LSA material may not be considered in determining the estimated average specific activity of the package contents. LSA material must be in one of three groups:
1. LSA-I
a. Uranium and thorium ores, concentrates of uranium and thorium ores, and other ores containing naturally occurring radioactive radionuclide radionuclides that are not intended to be processed for the use of these radionuclides;
b. Solid unirradiated natural Natural uranium or, depleted uranium or, natural thorium or their solid or liquid compounds or mixtures, provided they are unirradiated and in solid or liquid form;
c. Radioactive material other than fissile material, for which the A2 value is unlimited; or
d. Other radioactive material in which the activity is distributed throughout and the estimated average specific activity does not exceed 30 times the value for exempt material activity concentration determined in accordance with 12VAC5-481-3720.
2. LSA-II
a. Water with tritium concentration up to 0.8 terabecquerel per liter (20.0 Ci/L); or
b. Other radioactive material in which the activity is distributed throughout, and the estimated average specific activity does not exceed 1.0 E-04 A2/g for solids and gases, and 1.0 E-05 A2/g for liquids.
3. LSA-III
Solids (e.g., consolidated wastes, activated materials), excluding powders, that satisfy the requirements of 10 CFR 71.77) in which:
a. The radioactive material is distributed throughout a solid or a collection of solid objects, or is essentially uniformly distributed in a solid compact binding agent (e.g., concrete, bitumen, or ceramic);
b. The radioactive material is relatively insoluble, or it is intrinsically contained in a relatively insoluble material, so that, even under loss of packaging, the loss of radioactive material per package by leaching, when placed in water for seven days, would not exceed 0.1 A2; and
c. The estimated average specific activity of the solid, excluding any shielding material, does not exceed 2.0 E-03 A2/g.
"Low toxicity alpha emitters" means natural uranium, depleted uranium, natural thorium; uranium-235, uranium-238, thorium-232, thorium-228 or thorium-230 when contained in ores or physical or chemical concentrates or tailings; or alpha emitters with a half-life of less than 10 days.
"Lung class" (See "Class").
"mA" means milliampere.
"mAs" means milliampere second.
"Major processor" means a user processing, handling, or manufacturing radioactive material exceeding Type A quantities as unsealed sources or material, or exceeding four times Type B quantities as sealed sources, but does not include nuclear medicine programs, universities, industrial radiographers, or small industrial programs. Type A and B quantities are defined in this section.
"Management" means the chief executive officer or that individual's designee.
"MBq" means megabecquerels.
"Medical event" means an event that meets the criteria in 12VAC5-481-2080.
"Medical institution" means an organization in which several medical disciplines are practiced.
"Medical use" means the intentional internal or external administration of radioactive material or the radiation from radioactive material to patients or human research subjects under the supervision of an authorized user.
"Megavolt" or "MV" means the energy equal to that acquired by a particle with one electron charge in passing through a potential difference of one million volts in a vacuum. (Note: current convention is to use MV for photons and MeV for electrons.)
"Member of the public" means an individual except when that individual is receiving an occupational dose.
"Mineral logging" means any logging performed for the purpose of mineral exploration other than oil or gas.
"Minor" means an individual less than 18 years of age.
"Misadministration" means either:
1. An x-ray teletherapy radiation dose:
a. Involving the wrong patient;
b. Involving the wrong mode of treatment;
c. Involving the wrong treatment site;
d. Where the calculated total administered dose differs from the total prescribed dose by more than 10% when the treatment consists of three or fewer fractions;
e. Where the calculated weekly administered dose differs from the weekly prescribed dose by 30%; or
f. Where the calculated total administered dose differs from the total prescribed dose by more than 20%; or
2. An x-ray brachytherapy radiation dose:
a. Involving the wrong patient;
b. Involving the wrong treatment site; or
c. Where the calculated administered dose differs from the prescribed dose by more than 20%.
"mm" means millimeters.
"Mobile device" means a piece of equipment containing licensed radioactive materials that is either mounted on wheels or casters, or otherwise equipped for moving without a need for disassembly or dismounting, or designed to be hand carried. Mobile devices do not include stationary equipment installed in a fixed location.
"Mobile electronic brachytherapy service" means transportation of an electronic brachytherapy device to provide electronic brachytherapy at an address that is not the address of record.
"Mobile nuclear medicine service" means the transportation and medical use of radioactive material.
"Mobile x-ray equipment" (See "x-ray equipment").
"Mode of operation" means, for fluoroscopy systems, a distinct method of fluoroscopy or radiography provided by the manufacturer and selected with a set of several technique factors or other control settings uniquely associated with the mode. The set of distinct technique factors and control settings for the mode may be selected by the operation of a single control. Examples of distinct modes of operation include normal fluoroscopy (analog or digital), high-level control fluoroscopy, cineradiography (analog and digital), digital subtraction angiography, electronic radiography using the fluoroscopic image receptor, and photospot recording. In a specific mode of operation, certain system variables affecting kerma, AKR, or image quality, such as image magnification, x-ray field size, pulse rate, pulse duration, number of pulses, source-image receptor distance (SID), or optical aperture, may be adjustable or may vary; their variation per se does not comprise a mode of operation different from the one that has been selected.
"Monitor unit" or "MU" (See "Dose monitor unit").
"Monitoring" means the measurement of radiation, radioactive material concentrations, surface area activities or quantities of radioactive material and the use of the results of these measurements to evaluate potential exposures and doses. For purposes of these regulations, "radiation monitoring" and "radiation protection monitoring" are equivalent terms. For Part XI (12VAC5-481-2330 et seq.) of this chapter, it means observing and making measurements to provide data to evaluate the performance and characteristics of the disposal site.
"Movement control center" means an operation center that is remote from the transport activity and that maintains the position information on the movement of radioactive material, receives reports of attempted attacks or thefts, provides a means for reporting these and other problems to appropriate agencies and can request and coordinate appropriate aid.
"Moving beam radiation therapy" means radiation therapy with any planned displacement of radiation field or patient relative to each other, or with any planned change of absorbed dose distribution. It includes arc, skip, conformal, intensity modulation and rotational therapy.
"Multiple tomogram system" means a computed tomography x-ray system that obtains x-ray transmission data simultaneously during a single scan to produce more than one tomogram.
"NARM" means any naturally occurring or accelerator-produced radioactive material. It does not include byproduct, source, or special nuclear material.
"National Sealed Source and Device Registry" or "SSDR" means the national registry that contains the registration certificates, maintained by the NRC, that summarize the radiation safety information for sealed sources and devices, and describes the licensing and use conditions approved for the product.
"Nationally tracked source" means a sealed source containing a quantity equal to or greater than Category 1 or Category 2 levels of any radioactive material listed in 12VAC5-481-3780. In this context a sealed source is defined as radioactive material that is sealed in a capsule or closely bonded, in a solid form and that is not exempt from regulatory control. It does not mean material encapsulated solely for disposal, or nuclear material contained in any fuel assembly, subassembly, fuel rod, or fuel pellet. Category 1 nationally tracked sources are those containing radioactive material at a quantity equal to or greater than the Category 1 threshold. Category 2 nationally tracked sources are those containing radioactive material at a quantity equal to or greater than the Category 2 threshold but less than the Category 1 threshold.
"Natural radioactivity" means radioactivity of naturally occurring nuclides.
"Natural thorium" means thorium with the naturally occurring distribution of thorium isotopes, which is essentially 100 weight percent thorium-232.
"Natural uranium" (See "Uranium - natural, depleted, enriched").
"Near-surface disposal facility" means a land disposal facility in which waste is disposed of within approximately the upper 30 meters of the earth's surface.
"Negative pressure respirator" or "tight fitting" means a respirator in which the air pressure inside the facepiece is negative during inhalation with respect to the ambient air pressure outside the respirator.
"No-later-than arrival time" means the date and time that the shipping licensee and receiving licensee have established as the time at which an investigation will be initiated if the shipment has not arrived at the receiving facility. The no-later-than arrival times may not be more than six hours after the estimated arrival time for shipments of Category 2 quantities of radioactive material.
"Noise" means the standard deviation of the fluctuations in CTN expressed as a percentage of the attenuation coefficient of water. Its estimate (Sn) is calculated using the following expression:

where:
 = Linear attenuation coefficient of the material of interest.
= Linear attenuation coefficient of the material of interest.
µw = Linear attenuation coefficient of water.
s = Standard deviation of the CTN of picture elements in a specified area of the CT image.
"Nominal tomographic section thickness" means the full width at half-maximum of the sensitivity profile taken at the center of the cross-sectional volume over which x-ray transmission data are collected.
"Non-image-intensified fluoroscopy" means fluoroscopy using only a fluorescent screen.
"Nonstochastic effect" means a health effect, the severity of which varies with the dose and for which a threshold is believed to exist. Radiation-induced cataract formation is an example of a nonstochastic effect. For purposes of these regulations, "deterministic effect" is an equivalent term.
"NORM" means any naturally occurring radioactive material. It does not include accelerator produced, byproduct, source, or special nuclear material.
"Normal form radioactive material" means radioactive material that has not been demonstrated to qualify as special form radioactive material.
"Normal operating procedures" mean step-by-step instructions necessary to accomplish the analysis. These procedures shall include sample insertion and manipulation, equipment alignment, routine maintenance by the registrant (or licensee), and data recording procedures, which are related to radiation safety.
"Nominal treatment distance" means:
1. For electron irradiation, the distance from the scattering foil, virtual source, or exit window of the electron beam to the entrance surface of the irradiated object along the central axis of the useful beam.
2. For x-ray irradiation, the virtual source or target to isocenter distance along the central axis of the useful beam. For nonisocentric equipment, this distance shall be that specified by the manufacturer.
"NRC Forms 540, 540A, 541, 541A, 542, and 542A" means official NRC forms referenced in this chapter. Licensees need not use originals of these NRC Forms as long as any substitute forms are equivalent to the original documentation in respect to content, clarity, size, and location of information. Upon agreement between the shipper and consignee, NRC Forms 541 (and 541A) and NRC Forms 542 (and 542A) may be completed, transmitted, and stored in electronic media. The electronic media must have the capability for producing legible, accurate, and complete records in the format of the uniform manifest.
"Nuclear Regulatory Commission" or "NRC" means the NRC or its duly authorized representatives.
"Nuclear waste" means a quantity of source, byproduct or special nuclear material (the definition of nuclear waste in this chapter is used in the same way as in 49 CFR 173.403) required to be in NRC-approved specification packaging while transported to, through or across a state boundary to a disposal site, or to a collection point for transport to a disposal site.
"Occupational dose" means the dose received by an individual in the course of employment in which the individual's assigned duties for the licensee or registrant involve exposure to sources of radiation, whether or not the sources of radiation are in the possession of the licensee, registrant, or other person. Occupational dose does not include doses received from background radiation, from any medical administration the individual has received, from exposure to individuals administered radioactive material and released in accordance with 12VAC5-481-1870, from voluntary participation in medical research programs, or as a member of the public.
"Offshore platform radiography" means industrial radiography conducted from a platform over a body of water.
"Offshore waters" means that area of land and water, beyond the Commonwealth of Virginia's jurisdiction, on or above the U.S. Outer Continental Shelf.
"Open-beam configuration" means an analytical x-ray system in which an individual could accidentally place some part of his body in the primary beam path during normal operation.
"Output" means the exposure rate, dose rate, or a quantity related in a known manner to these rates from a teletherapy unit for a specified set of exposure conditions.
"Package" means the packaging together with its radioactive contents as presented for transport.
1. Fissile material package or Type AF package, Type BF package, Type B(U)F package, or Type B(M)F package means a fissile material packaging together with its fissile material contents.
2. Type A package means a Type A packaging together with its radioactive contents. A Type A package is defined and must comply with the DOT regulations in 49 CFR Part 173.
3. Type B package means a Type B packaging together with its radioactive contents. On approval, a Type B package design is designated by NRC as B(U) unless the package has a maximum normal operating pressure of more than 700 kPa (100 lbs/in2) gauge or a pressure relief device that would allow the release of radioactive material to the environment under the tests specified in 10 CFR 71.73 (hypothetical accident conditions), in which case it will receive a designation B(M). B(U) refers to the need for unilateral approval of international shipments; B(M) refers to the need for multilateral approval of international shipments. There is no distinction made in how packages with these designations may be used in domestic transportation. To determine their distinction for international transportation, see DOT regulations in 49 CFR Part 173. A Type B package approved before September 6, 1983, was designated only as Type B. Limitations on its use are specified in 10 CFR 71.19.
"Packaging" means the assembly of components necessary to ensure compliance with the packaging requirements of these regulations. It may consist of one or more receptacles, absorbent materials, spacing structures, thermal insulation, radiation shielding, and devices for cooling or absorbing mechanical shocks. The vehicle, tie-down system, and auxiliary equipment may be designated as part of the packaging.
"Panoramic dry-source-storage irradiator" means an irradiator in which the irradiations occur in air in areas potentially accessible to personnel and in which the sources are stored in shields made of solid materials. The term includes beam-type dry-source-storage irradiators in which only a narrow beam of radiation is produced for performing irradiations.
"Panoramic irradiator" means an irradiator in which the irradiations are done in air in areas potentially accessible to personnel. The term includes beam-type irradiators.
"Panoramic wet-source-storage irradiator" means an irradiator in which the irradiations occur in air in areas potentially accessible to personnel and in which the sources are stored under water in a storage pool.
"Particle accelerator" (See "Accelerator").
"Patient" means an individual or animal subjected to healing arts examination, diagnosis, or treatment.
"PBL" (See "Positive beam limitation").
"Peak tube potential" means the maximum value of the potential difference across the x-ray tube during an exposure.
"Periodic quality assurance check" means a procedure that is performed to ensure that a previous calibration continues to be valid.
"Permanent radiographic installation" means an enclosed shielded room, cell, or vault, not located at a temporary jobsite, in which radiography is performed.
"Person" means any individual, corporation, partnership, firm, association, trust, estate, public or private institution, group, department of the Commonwealth other than the Department of Health, political subdivision of the Commonwealth, any other state or political subdivision or department thereof, and any legal successor, representative, agent, or department of the foregoing, but not including federal government agencies.
"Personal supervision" means guidance and instruction by the supervisor who is physically present at the jobsite and watching the performance of the operation in such proximity that contact can be maintained and immediate assistance given as required. In radiography it means guidance and instruction provided to a radiographer trainee by a radiographer instructor who is present at the site, in visual contact with the trainee while the trainee is using sources of radiation, and in such proximity that immediate assistance can be given if required.
"Personnel monitoring equipment" (See "Individual monitoring devices").
"Phantom" means a volume of material behaving in a manner similar to tissue with respect to the attenuation and scattering of radiation. This requires that both the atomic number (Z) and the density of the material be similar to that of tissue.
"Physical description" means the items called for on NRC Form 541 to describe a low-level radioactive waste.
"Pool irradiator" means any irradiator at which the sources are stored or used in a pool of water including panoramic wet-source-storage irradiators and underwater irradiators.
"Pharmacist" means an individual licensed by this state to compound and dispense drugs, prescriptions, and poisons.
"Physician" means an individual licensed by this state to prescribe drugs in the practice of medicine.
"Picture element" means an elemental area of a tomogram.
"PID" (See "Position indicating device").
"Pigtail" (See "Source assembly").
"Pill" (See "Sealed source").
"Planned special exposure" means an infrequent exposure to radiation, separate from and in addition to the annual occupational dose limits.
"Portable x-ray equipment" (See "x-ray equipment").
"Position indicating device" means a device on dental x-ray equipment used to indicate the beam position and to establish a definite source-surface (skin) distance. It may or may not incorporate or serve as a beam-limiting device.
"Positive beam limitation" means the automatic or semi-automatic adjustment of an x-ray beam to the size of the selected image receptor, whereby exposures cannot be made without such adjustment.
"Positron emission tomography radionuclide production facility" or "PET" means a facility operating a cyclotron or other particle accelerator for the purpose of producing radionuclides that decay by positron emission.
"Positive pressure respirator" means a respirator in which the pressure inside the respiratory inlet covering exceeds the ambient air pressure outside the respirator.
"Powered air-purifying respirator" or "PAPR" means an air-purifying respirator that uses a blower to force the ambient air through air-purifying elements to the inlet covering.
"Practical examination" means a demonstration through application of the safety rules and principles in industrial radiography including use of all procedures and equipment to be used by radiographic personnel.
"Practical range of electrons" corresponds to classical electron range where the only remaining contribution to dose is from bremsstrahlung x-rays. A further explanation may be found in "Clinical Electron Beam Dosimetry: Report of AAPM Radiation Therapy Committee Task Group 25" (Medical Physics 18(1): 73-109, Jan/Feb. 1991) and ICRU Report 35, "Radiation Dosimetry: Electron Beams with Energies Between 1 and 50 MeV", International Commission on Radiation Units and Measurements, September 15, 1984.
"Preceptor" means an individual who provides, directs, or verifies training and experience required for an individual to become an authorized user, an authorized medical physicist, an authorized nuclear pharmacist, or a radiation safety officer.
"Prescribed dosage" means the quantity of radiopharmaceutical activity as documented:
1. In a written directive; or
2. Either in the diagnostic clinical procedures manual or in any appropriate record in accordance with the directions of the authorized user for diagnostic procedures.
"Prescribed dose" means:
1. For gamma stereotactic radiosurgery, the total dose as documented in the written directive;
2. For teletherapy, the total dose and dose per fraction as documented in the written directive. The prescribed dose is an estimation from measured data from a specified therapeutic machine using assumptions that are clinically acceptable for that treatment technique and historically consistent with the clinical calculations previously used for patients treated with the same clinical technique; or
3. For brachytherapy, either the total source strength and exposure time, or the total dose, as documented in the written directive.
"Pressure demand respirator" means a positive pressure atmosphere-supplying respirator that admits breathing air to the facepiece when the positive pressure is reduced inside the facepiece by inhalation.
"Primary beam" means radiation that passes through an aperture of the source housing by a direct path from the x-ray tube or a radioactive source located in the radiation source housing.
"Primary dose monitoring system" means a system that will monitor the useful beam during irradiation and that will terminate irradiation when a preselected number of dose monitor units have been delivered.
"Primary protective barrier" means the material, excluding filters, placed in the useful beam to reduce the radiation exposure (beyond the patient and cassette holder) for protection barriers.
"Principal activities," as used in this chapter, means activities authorized by the license that are essential to achieving the purposes for which the license was issued or amended. Storage during which no licensed material is accessed for use or disposal and activities incidental to decontamination or decommissioning are not principal activities.
"Private inspector" means an individual who meets the requirements set forth in 12VAC5-481-340 and who has demonstrated to the satisfaction of the agency that such individual possesses the knowledge, training and experience to measure ionizing radiation, to evaluate safety techniques, and to advise regarding radiation protection needs.
"Product" means, as used in Part XVI (12VAC5-481-3460 et seq.) of this chapter, something produced, made, manufactured, refined, or benefited.
"Product conveyor system" means a system for moving the product to be irradiated to, from, and within the area where irradiation takes place.
"Projection sheath" (See "Guide tube").
"Projector" (See "Radiographic exposure device").
"Protective apron" means an apron made of radiation-attenuating or absorbing materials used to reduce exposure to radiation.
"Protective glove" means a glove made of radiation absorbing materials used to reduce radiation exposure.
"Public dose" means the dose received by a member of the public from exposure to sources of radiation released by the licensee or registrant, or to any other source of radiation under the control of the licensee or registrant. "Public dose" does not include occupational dose, or doses received from background radiation, from any medical administration the individual has received, from exposure to individuals administered radioactive material and released in accordance with 12VAC5-481-1870, or from voluntary participation in medical research programs.
"Pulsed mode" means operation of the x-ray system such that the x-ray tube is pulsed by the x-ray control to produce one or more exposure intervals of duration less than one-half second.
"Pyrophoric material" means any liquid that ignites spontaneously in dry or moist air at or below 130°F (54.4°C) or any solid material, other than one classed as an explosive, which under normal conditions is liable to cause fires through friction, retained heat from manufacturing or processing, or that can be ignited readily and, when ignited, burns so vigorously and persistently as to create a serious transportation, handling, or disposal hazard. Included are spontaneously combustible and water-reactive materials.
"Qualified inspector" means an individual who is granted professional privileges based on education and experience to provide clinical services in diagnostic and therapeutic medical physics.
"Qualified medical physicist" means an individual qualified in accordance with 12VAC5-481-3390 D.
"Qualitative fit test" or "QLFT" means a pass/fail fit test to assess the adequacy of respirator fit that relies on the individual's response to the test agent.
"Quality factor" or "Q" means the modifying factor, that is referenced in 12VAC5-481-240, that is used to derive dose equivalent from absorbed dose.
"Quantitative fit test" or "QNFT" means an assessment of the adequacy of respirator fit by numerically measuring the amount of leakage into the respirator.
"Quarter" means a period of time equal to one-fourth of the year observed by the licensee, approximately 13 consecutive weeks, providing that the beginning of the first quarter in a year coincides with the starting date of the year and that no day is omitted or duplicated in consecutive quarters.
"Rad" means the special unit of absorbed dose. One rad is equal to an absorbed dose of 100 erg per gram or 0.01 joule per kilogram (0.01 gray).
"Radiation" means alpha particles, beta particles, gamma rays, x-rays, neutrons, high-speed electrons, high-speed protons, and other particles capable of producing ions. For purposes of these regulations, ionizing radiation is an equivalent term. Radiation, as used in these regulations, does not include nonionizing radiation, such as radiowaves or microwaves, visible, infrared, or ultraviolet light.
"Radiation area" means any area, accessible to individuals, in which radiation levels could result in an individual receiving a dose equivalent in excess of 0.05 mSv (0.005 rem) in one hour at 30 centimeters from the source of radiation or from any surface that the radiation penetrates.
"Radiation dose" (See "Dose").
"Radiation field" (See "Useful beam").
"Radiation head" means the structure from which the useful beam emerges.
"Radiation machine" means any device capable of producing radiation except those devices with radioactive material as the only source of radiation.
"Radiation room" means a shielded room in which irradiations take place. Underwater irradiators do not have radiation rooms.
"Radiation safety officer" or "RSO" means an individual who has the knowledge and responsibility to apply appropriate radiation protection regulations and has been assigned such responsibility by the licensee or registrant.
"Radiation safety officer for industrial radiography" means an individual with the responsibility for the overall radiation safety program on behalf of the licensee or registrant and who meets the requirements of 12VAC5-481-1310.
"Radiation safety officer for medical" means an individual who meets the requirements of 12VAC5-481-1750 and 12VAC5-481-1790 or is identified as an RSO on: a medical use license issued by the agency, NRC or another agreement state, or a medical use permit issued by an NRC masters material licensee.
"Radiation therapy physicist" means an individual qualified in accordance with 12VAC5-481-340.
"Radiation therapy simulation system" means a radiographic or fluoroscopic x-ray system intended for localizing the volume to be exposed during radiation therapy and confirming the position and size of the therapeutic irradiation field.
"Radiation therapy system" means a device that delivers radiation to a specific area of the body where cancer cells or tumors are located.
"Radioactive material" means any solid, liquid, or gas which emits radiation spontaneously.
"Radioactive marker" means radioactive material placed subsurface or on a structure intended for subsurface use for the purpose of depth determination or direction orientation.
"Radioactivity" means the transformation of unstable atomic nuclei by the emission of radiation.
"Radiobioassay" (See "Bioassay").
"Radiograph" means an image receptor on which the image is created directly or indirectly by an x-ray pattern and results in a permanent record.
"Radiographer" means any individual who performs or who, in attendance at the site where the sources of radiation are being used, personally supervises industrial radiographic operations and who is responsible to the licensee or registrant for assuring compliance with the requirements of the agency's regulations and the conditions of the license or registration.
"Radiographer certification" means written approval received from a certifying entity stating that an individual has satisfactorily met the radiation safety, testing, and experience criteria in 12VAC5-481-1320.
"Radiographer instructor" means any radiographer who has been authorized by the agency to provide on-the-job training to radiographer trainees in accordance with Part V (12VAC5-481-1170 et seq.) of this chapter.
"Radiographer trainee" means any individual who, under the personal supervision of a radiographer instructor, uses sources of radiation, related handling tools, or radiation survey instruments during the course of his instruction.
"Radiographer's assistant" means any individual who under the direct supervision of a radiographer, uses radiographic exposure devices, sources of radiation, related handling tools, or radiation survey instruments in industrial radiography.
"Radiographic exposure device" means any instrument containing a sealed source fastened or contained therein, in which the sealed source or shielding thereof may be moved, or otherwise changed, from a shielded to unshielded position for purposes of making a radiographic exposure.
"Radiographic operations" means all activities performed with a radiographic exposure device, or with a radiation machine. Activities include using, transporting except by common or contract carriers, or storing at a temporary job site, performing surveys to confirm the adequacy of boundaries, setting up equipment, and any activity inside restricted area boundaries. Transporting a radiation machine is not considered a radiographic operation.
"Radiographic personnel" means any radiographer, radiographer instructor, or radiographer trainee.
"Radiography" means:
1. For radioactive materials: See "Industrial radiography."
2. For x-ray: A technique for generating and recording an x-ray pattern for the purpose of providing the user with an image after termination of the exposure.
"Rating" means the operating limits as specified by the component manufacturer.
"Reasonably maximally exposed individual" means, as used in Part XVI (12VAC5-481-3460 et seq.) of this chapter, a representative of a population who is exposed to TENORM at the maximum TENORM concentration measured in environmental media found at a site along with reasonable maximum case exposure assumptions. The exposure is determined by using maximum values for one or more of the most sensitive parameters affecting exposure, based on cautious but reasonable assumptions, while leaving the others at their mean value.
"Recording" means producing a retrievable form of an image resulting from x-ray photons.
"Redundant beam monitoring system" means a combination of two dose monitoring systems in which each system is designed to terminate irradiation in accordance with a preselected number of dose monitor units.
"Reference man" means a hypothetical aggregation of human physical and physiological characteristics determined by international consensus. These characteristics may be used by researchers and public health employees to standardize results of experiments and to relate biological insult to a common base. A description of the reference man is contained in the International Commission on Radiological Protection report, ICRP Publication 23, "Report of the Task Group on Reference Man."
"Reference plane" means a plane that is displaced from and parallel to the tomographic plane.
"Registrant" means any person who is registered with the agency and is legally obligated to register with the agency pursuant to these regulations and the Act.
"Registration" means registration with the agency in accordance with the regulations adopted by the agency.
"Regulations of the U.S. Department of Transportation" means the regulations in 49 CFR Parts 100 - 189.
"Rem" means the special unit of any of the quantities expressed as dose equivalent. The dose equivalent in rems is equal to the absorbed dose in rad multiplied by the quality factor (1 rem = 0.01 Sv).
"Reportable event" means the administration of either:
1. A diagnostic x-ray exposure where an actual or suspected acute or long-term functional damage to an organ or a physiological system has occurred. Exempt from this reporting requirement is any event when any functional damage to a patient organ or a physiological system that was an expected outcome when the causative procedures were prescribed;
2. A procedure where the patient or operator is injured as a result of a mechanical injury;
3. A teletherapy x-ray or electron dose where the calculated weekly administered dose differs from the weekly prescribed dose by 15% or more; or
4. A brachytherapy x-ray dose where the calculated administered dose differs from the prescribed dose by 10% or more.
"Research and development" means (i) theoretical analysis, exploration, or experimentation; or (ii) the extension of investigative findings and theories of a scientific or technical nature into practical application for experimental and demonstrative purposes, including the experimental production and testing of models, devices, equipment, materials, and processes. Research and development does not include the internal or external administration of radiation or radioactive material to human beings.
"Residential location" means any area where structures in which people lodge or live are located, and the grounds on which such structures are located including, but not limited to, houses, apartments, condominiums, and garages.
"Residual radioactive material" means (i) waste (that the U.S. Secretary of Energy determines to be radioactive) in the form of tailings resulting from the processing of ores for the extraction of uranium and other valuable constituents of the ores and (ii) other waste (that the U.S. Secretary of Energy determines to be radioactive) at a processing site that relates to such processing, including any residual stock of unprocessed ores or low-grade materials. This term is used only with respect to materials at sites subject to remediation under Title I of the Uranium Mill Tailings Radiation Control Act of 1978, as amended.
"Residual radioactivity" means radioactivity in structures, materials, soils, groundwater, and other media at a site resulting from activities under the licensee's control. This includes radioactivity from all licensed and unlicensed sources used by the licensee, but excludes background radiation. It also includes radioactive materials remaining at the site as a result of routine or accidental releases of radioactive materials at the site and previous burials at the site, even if those burials were made in accordance with the provisions of Part IV (12VAC5-481-600 et seq.) of this chapter.
"Residual waste" means low-level radioactive waste resulting from processing or decontamination activities that cannot be easily separated into distinct batches attributable to specific waste generators. This waste is attributable to the processor or decontamination facility, as applicable.
"Respiratory protective device" means an apparatus, such as a respirator, used to reduce an individual's intake of airborne radioactive materials.
"Restricted area" means an area, access to which is limited by the licensee or registrant for the purpose of protecting individuals against undue risks from exposure to radiation and radioactive materials. Restricted area does not include areas used as residential quarters, but separate rooms in a residential building may be set apart as a restricted area.
"Reviewing official" means the individual who shall make the trustworthiness and reliability determination of an individual to determine whether the individual may have, or continue to have, unescorted access to the Category 1 or Category 2 quantities of radioactive materials that are possessed by the licensee.
"Roentgen" means the special unit of exposure. One roentgen (R) equals 2.58E-4 coulombs per kilogram of air (see "Exposure" and 12VAC5-481-240).
"S-tube" means a tube through which the radioactive source travels when inside a radiographic exposure device.
"Sabotage" means deliberate damage, with malevolent intent, to a Category 1 or Category 2 quantity of radioactive material, a device that contains a Category 1 or Category 2 quantity of radioactive material, or the components of the security system.
"Safe haven" means a readily recognizable and readily accessible site at which security is present or from which, in the event of an emergency, the transport crew can notify and wait for the local law-enforcement authorities.
"Sanitary sewerage" means a system of public sewers for carrying off waste water and refuse, but excluding sewage treatment facilities, septic tanks, and leach fields owned or operated by the licensee or registrant.
"Scan" means the complete process of collecting x-ray transmission data for the production of a tomogram. Data can be collected simultaneously during a single scan for the production of one or more tomograms.
"Scan increment" means the amount of relative displacement of the patient with respect to the CT x-ray system between successive scans measured along the direction of such displacement.
"Scan sequence" means a preselected set of two or more scans performed consecutively under preselected CT conditions of operation.
"Scan time" means the period of time between the beginning and end of x-ray transmission data accumulation for a single scan.
"Scattered radiation" means ionizing radiation emitted by interaction of ionizing radiation with matter, the interaction being accompanied by a change in direction of the radiation. Scattered primary radiation means that scattered radiation which has been deviated in direction only by materials irradiated by the useful beam.
"Sealed source" means any radioactive material that is encased in a capsule designed to prevent leakage or escape of any radioactive material.
"Secondary dose monitoring system" means a system which will terminate irradiation in the event of failure of the primary dose monitoring system.
"Security zone" means any temporary or permanent area determined and established by the licensee for the physical protection of Category 1 or Category 2 quantities of radioactive material.
"Seismic area" means any area where the probability of a horizontal acceleration in rock of more than 0.3 times the acceleration of gravity in 250 years is greater than 10%, as designated by the United States Geological Survey.
"Self-contained breathing apparatus" or "SCBA" means an atmosphere-supplying respirator for which the breathing air source is designed to be carried by the user.
"Shadow tray" means a device attached to the radiation head to support auxiliary beam blocking material.
"Shallow dose equivalent" or "Hs," which applies to the external exposure of the skin or an extremity, means the dose equivalent at a tissue depth of 0.007 centimeter (7 mg/cm2).
"Shielded position" means the location within the radiographic exposure device or storage container which, by manufacturer's design, is the proper location for storage of the sealed source.
"Shielded-room radiography" means industrial radiography conducted in a room shielded so that radiation levels at every location on the exterior meet the limitations specified in 12VAC5-481-640.
"Shipper" means the licensed entity (i.e., the waste generator, waste collector, or waste processor) who offers low-level radioactive waste for transportation, typically consigning this type of waste to a licensed waste collector, waste processor, or land disposal facility operator.
"Shipping paper" means NRC Form 540 and, if required, NRC Form 540A, which includes the information required by the U.S. Department of Transportation in 49 CFR Part 172.
"Shutter" means a device attached to the tube housing assembly which can intercept the entire cross sectional area of the useful beam and which has a lead equivalency not less than that of the tube housing assembly.
"SI" means the abbreviation for the International System of Units.
"SID" (See "Source-image receptor distance").
"Sievert" or "Sv" means the SI unit of any of the quantities expressed as dose equivalent. The dose equivalent in sievert is equal to the absorbed dose in gray multiplied by the quality factor (1 Sv = 100 rem).
"Simulator" or "radiation therapy simulation system" means any x-ray system intended for localizing the volume to be exposed during radiation therapy and reproducing the position and size of the therapeutic irradiation field.
"Single tomogram system" means a CT x-ray system that obtains x-ray transmission data during a scan to produce a single tomogram.
"Site area emergency" means events may occur, are in progress, or have occurred that could lead to a significant release of radioactive material and that could require a response by offsite response organizations to protect persons offsite.
"Site boundary" means that line beyond which the land or property is not owned, leased, or otherwise controlled by the licensee.
"Site closure and stabilization" means those actions that are taken upon completion of operations that prepare the disposal site for custodial care and that assure that the disposal site will remain stable and will not need ongoing active maintenance.
"Source" means the focal spot of the x-ray tube.
"Source assembly" means an assembly that consists of the sealed source and a connector that attaches the source to the control cable. The source assembly may include a ballstop to secure the source in the shielded position.
"Source changer" means a device designed and used for replacement of sealed sources in radiographic exposure devices, including those source changers also used for transporting and storage of sealed sources.
"Source holder" means a housing or assembly into which a radioactive source is placed for the purpose of facilitating the handling and use of the source in well-logging operations.
"Source-image receptor distance" means the distance from the source to the center of the input surface of the image receptor.
"Source material" means:
1. Uranium or thorium, or any combination thereof, in any physical or chemical form; or
2. Ores that contain by weight one-twentieth of 1.0% (0.05%) or more of uranium, thorium or any combination of uranium and thorium. Source material does not include special nuclear material.
"Source of radiation" means any radioactive material or any device or equipment emitting, or capable of producing, radiation.
"Source-skin distance" or "SSD" means the distance from the source to the center of the entrant x-ray field in the plane tangent to the patient's skin surface.
"Source traceability" means the ability to show that a radioactive source has been calibrated either by the national standards laboratory of the National Institute of Standards and Technology, or by a laboratory that participates in a continuing measurement quality assurance program with National Institute of Standards and Technology or other equivalent national or international program.
"Special form radioactive material" means radioactive material that satisfies the following conditions:
1. It is either a single solid piece or is contained in a sealed capsule that can be opened only by destroying the capsule;
2. The piece or capsule has at least one dimension not less than five millimeters (0.2 in.); and
3. It satisfies the test requirements specified by the NRC of 10 CFR 71.75. A special form encapsulation designed in accordance with the NRC requirements of 10 CFR 71.4 in effect on June 30, 1983 (see 10 CFR Part 71, revised as of January 1, 1983), and constructed prior to before July 1, 1985,; a special form encapsulation designed in accordance with the requirements of 10 CFR 71.4 in effect on March 31, 1996 (see 10 CFR Part 71, revised as of January 1, 1996), and constructed before April 1, 1998; and special form material that was successfully tested before September 10, 2015, in accordance with the requirements of 10 CFR 71.75(d) in effect before September 10, 2015, may continue to be used. A Any other special form encapsulation either designed or constructed after April 1, 1998, must meet requirements of this definition applicable at the time of its design or construction.
"Special nuclear material" means:
1. Plutonium, uranium-233, uranium enriched in the isotope 233 or in the isotope 235, and any other material the NRC, pursuant to the provisions of § 51 of the Atomic Energy Act of 1954, as amended, (42 USC § 2071) determines to be special nuclear material, but does not include source material; or
2. Any material artificially enriched by any of the foregoing but does not include source material.
"Special nuclear material in quantities not sufficient to form a critical mass" means uranium enriched in the isotope U-235 in quantities not exceeding 350 grams of contained U-235; uranium-233 in quantities not exceeding 200 grams; plutonium in quantities not exceeding 200 grams; or any combination of them in accordance with the following formula: For each kind of special nuclear material, determine the ratio between the quantity of that special nuclear material and the quantity specified above for the same kind of special nuclear material. The sum of such ratios for all of the kinds of special nuclear material in combination shall not exceed 1. For example, the following quantities in combination would not exceed the limitation and are within the formula:
(175 grams contained U235/350) + (50 grams U – 233/200) + (50 grams Pu/200) = 1
"Specific activity of a radionuclide" means the radioactivity of a radionuclide per unit mass of that nuclide. The specific activity of a material in which the radionuclide is essentially uniformly distributed is the radioactivity per unit mass of the material.
"Spot film" means a radiograph that is made during a fluoroscopic examination to permanently record conditions that exist during that fluoroscopic procedure.
"Spot-film device" means a device intended to transport or position a radiographic image receptor between the x-ray source and fluoroscopic image receptor. It includes a device intended to hold a cassette over the input end of an image intensifier for the purpose of making a radiograph.
"Stability" means structural stability.
"State inspector" means an employee of the Virginia Department of Health designated to perform those duties or functions assigned the Radiological Health Program.
"Stationary beam radiation therapy" means radiation therapy without displacement of one or more mechanical axes relative to the patient during irradiation.
"Stationary x-ray equipment" (See "x-ray equipment").
"Stochastic effect" means a health effect that occurs randomly and for which the probability of the effect occurring, rather than its severity, is assumed to be a linear function of dose without threshold. Hereditary effects and cancer incidence are examples of stochastic effects. For purposes of this chapter, "probabilistic effect" is an equivalent term.
"Storage" means a condition in which a device or source is not being used for an extended period of time, and has been made inoperable.
"Storage area" means any location, facility, or vehicle that is used to store and secure a radiographic exposure device, a radiation machine, or a storage container when it is not used for radiographic operations. Storage areas are locked or have a physical barrier to prevent accidental exposure, tampering, or unauthorized removal of the device, machine, or container.
"Storage container" means a device in which sealed sources or radiation machines are secured and stored.
"Stray radiation" means the sum of leakage and scattered radiation.
"Subsurface tracer study" means the release of a substance tagged with radioactive material for the purpose of tracing the movement or position of the tagged substance in the well-bore or adjacent formation.
"Supplied-air respirator," "airline respirator," or "SAR" means an atmosphere-supplying respirator for which the source of breathing air is not designed to be carried by the user.
"Surface contaminated object" or "SCO" means a solid object that is not itself classed as radioactive material, but that has radioactive material distributed on any of its surfaces. An SCO must be in one of two groups with surface activity not exceeding the following limits:
1. SCO-I: A solid object on which:
a. The nonfixed contamination on the accessible surface averaged over 300 cm², or the area of the surface if less than 300 cm², does not exceed four becquerel per cm² (1 E-04 µCi/cm²) for beta and gamma and low toxicity alpha emitters, or 0.4 becquerel per cm² (1 E-05 µCi/cm²) for all other alpha emitters;
b. The fixed contamination on the accessible surface averaged over 300 cm², or the area of the surface if less than 300 cm², does not exceed 4 E+04 becquerel per cm² (1.0 µCi/cm²) for beta and gamma and low toxicity alpha emitters, or 4 E+03 becquerel per cm² (0.1 µCi/cm²) for all other alpha emitters; and
c. The nonfixed contamination plus the fixed contamination on the inaccessible surface averaged over 300 cm², or the area of the surface if less than 300 cm², does not exceed 4 E+04 becquerel per cm² (1 µCi/cm²) for beta and gamma and low toxicity alpha emitters, or 4 E+03 Becquerel per cm² (0.1 µCi/cm²) for all other alpha emitters.
2. SCO-II: A solid object on which the limits for SCO-I are exceeded and on which:
a. The nonfixed contamination on the accessible surface averaged over 300 cm², or the area of the surface if less than 300 cm², does not exceed 400 becquerel per cm² (1 E-02 µCi/cm²) for beta and gamma and low toxicity alpha emitters or 40 becquerel per cm² (1 E-03 µCi/cm²) for all other alpha emitters;
b. The fixed contamination on the accessible surface averaged over 300 cm², or the area of the surface if less than 300 cm², does not exceed 8 E+05 becquerel per cm² (20 µCi/cm²) for beta and gamma and low toxicity alpha emitters, or 8 E+04 becquerel per cm² (2 µCi/cm²) for all other alpha emitters; and
c. The nonfixed contamination plus the fixed contamination on the inaccessible surface averaged over 300 cm², or the area of the surface if less than 300 cm², does not exceed 8 E+05 becquerel per cm² (20 µCi/cm²) for beta and gamma and low toxicity alpha emitters, or 8 E+04 becquerel per cm² (2 µCi/cm²) for all other alpha emitters.
"Surveillance" means monitoring and observation of the disposal site for purposes of visual detection of need for maintenance, custodial care, evidence of intrusion, and compliance with other license and regulatory requirements.
"Survey" means an evaluation of the radiological conditions and potential hazards incident to the production, use, transfer, release, disposal, or presence of radioactive material or other sources of radiation. When appropriate, such an evaluation includes a physical survey of the location of radioactive material and measurements or calculations of levels of radiation, or concentrations or quantities of radioactive material present.
"Tabletop, stationary" means a tabletop that, when assembled for use, is incapable of movement with respect to its supporting structure within the plane of the tabletop.
"Target" means that part of an x-ray tube or accelerator onto which a beam of accelerated particles is directed to produce ionizing radiation or other particles.
"Target-skin distance" or "TSD" means the distance measured along the beam axis from the center of the front surface of the x-ray target or electron virtual source, or both, to the surface of the irradiated object or patient.
"Technologically enhanced naturally occurring radioactive material" or "TENORM" means, as used in Part XVI (12VAC5-481-3460 et seq.) of this chapter, naturally occurring radionuclides whose concentrations are increased by or as a result of past or present human practices. TENORM does not include background radiation or the natural radioactivity of rocks or soils. TENORM does not include uranium or thorium in "source material" as defined in the AEA and NRC regulations.
"Technique factors" means the following conditions of operation:
1. For capacitor energy storage equipment, peak tube potential in kilovolts (kV) and quantity of charge in milliampere-seconds (mAs);
2. For field emission equipment rated for pulsed operation, peak tube potential in kilovolts (kV), and number of x-ray pulses;
3. For CT equipment designed for pulsed operation, peak tube potential in kilovolts (kV), scan time in seconds, and either tube current in milliamperes (mA), x-ray pulse width in seconds, and the number of x-ray pulses per scan, or the product of tube current, x-ray pulse width, and the number of x-ray pulses in milliampere-seconds (mAs);
4. For CT equipment not designed for pulsed operation, peak tube potential in kilovolts (kV), and either tube current in milliamperes (mA) and scan time in seconds, or the product of tube current and exposure time in milliampere-seconds (mAs) and the scan time when the scan time and exposure time are equivalent; and
5. For all other equipment, peak tube potential in kilovolts (kV), and either tube current in milliamperes (mA) and exposure time in seconds, or the product of tube current and exposure time in milliampere-seconds (mAs).
"Telemetric position monitoring system" means a data transfer system that captures information by either instrumentation or measuring devices, or both, about the location and status of a transport vehicle or package between the departure and destination locations.
"Teletherapy physicist" means an individual identified as a qualified teletherapy physicist on an agency license.
"Teletherapy" means therapeutic irradiation in which the source of radiation is at a distance from the body.
"Temporary job site" means any location where industrial radiography, wireline service, well-logging, portable gauge or x-ray fluorescence use is performed and where licensed material may be stored other than those locations of use authorized on the license.
"Tenth-value layer" or "TVL" means the thickness of a specified material that attenuates x-radiation or gamma radiation to an extent such that the air kerma rate, exposure rate, or absorbed dose rate is reduced to one-tenth of the value measured without the material at the same point.
"Test" means the process of verifying compliance with an applicable regulation.
"Therapeutic radiation machine" means x-ray or electron-producing equipment designed and used for external beam radiation therapy. For the purpose of this chapter, devices used to administer electronic brachytherapy shall also be considered therapeutic radiation machines.
"These regulations" mean all parts of this chapter.
"Tight-fitting facepiece" means a respiratory inlet covering that forms a complete seal with the face.
"Tomogram" means the depiction of the x-ray attenuation properties of a section through the body.
"Tomographic plane" means that geometric plane that is identified as corresponding to the output tomogram.
"Tomographic section" means the volume of an object whose x-ray attenuation properties are imaged in a tomogram.
"Total effective dose equivalent" or "TEDE" means the sum of the effective dose equivalent for external exposures and the committed effective dose equivalent for internal exposures.
"Total organ dose equivalent" or "TODE" means the sum of the deep dose equivalent and the committed dose equivalent to the organ receiving the highest dose as described in 12VAC5-481-1040.
"Traceable to a National Standard" (See "Instrument traceability" or "Source traceability").
"Transfer" means, as used in Part XVI (12VAC5-481-3460 et seq.) of this chapter, the physical relocation of NORM containing materials not directly associated with commercial distribution within a business's operation or between general or specific licensees. This term does not include a change in legal title to NORM containing materials that does not involve physical movement of those materials.
"Transport container" means a package that is designed to provide radiation safety and security when sealed sources are transported and that meets all applicable requirements of the U.S. Department of Transportation.
"Transport index" or "TI" means the dimensionless number, rounded up to the next tenth, placed on the label of a package to designate the degree of control to be exercised by the carrier during transportation. The transport index is the number determined by multiplying the maximum radiation level in millisievert (mSv) per hour at one meter (3.3 feet) from the external surface of the package by 100 (equivalent to the maximum radiation level in millirem per hour at one meter (3.3 feet)).
"Treatment site" means the correct anatomical description of the area intended to receive a radiation dose, as described in a written directive.
"Tribal official" means the highest ranking individual that represents tribal leadership, such as the chief, president, or tribal council leadership.
"Tritium neutron generator target source" means a tritium source used within a neutron generator tube to produce neutrons for use in well-logging applications.
"Trustworthiness and reliability" means characteristics of an individual considered dependable in judgment, character, and performance, such that unescorted access to Category 1 or Category 2 quantities of radioactive material by that individual does not constitute an unreasonable risk to the public health and safety or security. A determination of trustworthiness and reliability for this purpose is based upon the results from a background investigation.
"Tube" means an x-ray tube, unless otherwise specified.
"Tube housing assembly" means the tube housing with tube installed. It includes high-voltage or filament transformers and other appropriate elements when such are contained within the tube housing.
"Tube rating chart" means the set of curves which specify the rated limits of operation of the tube in terms of the technique factors.
"Type A quantity" means a quantity of radioactive material, the aggregate radioactivity of which does not exceed A1 for special form radioactive material or A2 for normal form radioactive material, where A1 and A2 are given in Table 1 of 12VAC5-481-3770 F or may be determined by procedures described in 12VAC5-481-3770 A through E.
"Type B quantity" means a quantity of radioactive material greater than a Type A quantity.
"Underwater irradiator" means an irradiator in which the sources always remain shielded under water and humans do not have access to the sealed sources or the space subject to irradiation without entering the pool.
"Underwater radiography" means radiographic operations performed when the radiographic exposure device or radiation machine or related equipment are beneath the surface of the water.
"Unescorted access" means solitary access to an aggregated Category 1 or Category 2 quantity of radioactive material or the devices that contain the material.
"Uniform Low-Level Radioactive Waste Manifest" or "uniform manifest" means the combination of NRC Forms 540 and 541, and, if necessary, 542, and their respective continuation sheets as needed, or equivalent.
"Unirradiated uranium" means uranium containing not more than 2 x 103 Bq of plutonium per gram of uranium-235, not more than 9 x 106 Bq of fission products per gram of uranium-235, and not more than 5 x 10-3 g of uranium-236 per gram of uranium-235.
"Unrefined and unprocessed ore" means ore in its natural form prior to any processing, such as grinding, roasting, beneficiating, or refining. Processing does not include sieving or encapsulating of ore or preparation of samples for laboratory analysis.
"Unrestricted area" means an area, access to which is neither limited nor controlled by the licensee or registrant. For purposes of these regulations, "uncontrolled area" is an equivalent term.
"Uranium - natural, depleted, enriched"
1. "Natural uranium" means uranium (which may be chemically separated) with the naturally occurring distribution of uranium isotopes, which is approximately 0.711 weight percent uranium-235, and the remainder by weight essentially uranium-238.
2. "Depleted uranium" means uranium containing less uranium-235 than the naturally occurring distribution of uranium isotopes.
3. "Enriched uranium" means uranium containing more uranium-235 than the naturally occurring distribution of uranium isotopes.
"Uranium sinker bar" means a weight containing depleted uranium used to pull a logging tool down toward the bottom of a well.
"Useful beam" means the radiation that passes through the tube housing port and the aperture of the beam-limiting device when the exposure switch or timer is activated.
"User seal check" or "fit check" means an action conducted by the respirator user to determine if the respirator is properly seated to the face. Examples include negative pressure check, positive pressure check, irritant smoke check, or isoamyl acetate check.
"Variable-aperture beam-limiting device" means a beam-limiting device which has capacity for stepless adjustment of the x-ray field size at a given SID.
"Very high radiation area" means an area, accessible to individuals, in which radiation levels from radiation sources external to the body could result in an individual receiving an absorbed dose in excess of five Gy (500 rad) in one hour at one meter from a source of radiation or one meter from any surface that the radiation penetrates.
"Virtual simulator" means a computed tomography (CT) unit used in conjunction with relevant software that recreates the treatment machine and that allows import, manipulation, display, and storage of images from CT or other imaging modalities, or both.
"Virtual source" means a point from which radiation appears to originate.
"Visible area" means that portion of the input surface of the image receptor over which incident x-ray photons are producing a visible image.
"Visiting authorized user" means an authorized user who is not identified on the license of the licensee being visited.
"Waste" means those low-level radioactive wastes containing source, special nuclear, or byproduct material that are acceptable for disposal in a land disposal facility. For the purposes of this definition, low-level radioactive waste means radioactive waste not classified as high-level radioactive waste, transuranic waste, spent nuclear fuel, or byproduct material as defined in subdivisions 2, 3, and 4 of the definition of byproduct material.
"Waste collector" means an entity, operating under a specific license, whose principal purpose is to collect and consolidate waste generated by others, and to transfer this waste, without processing or repackaging the collected waste, to another licensed waste collector, licensed waste processor, or licensed land disposal facility.
"Waste description" means the physical, chemical and radiological description of a low-level radioactive waste as called for on NRC Form 541.
"Waste generator" means an entity, operating under a license, that (i) possesses any material or component that contains radioactivity or is radioactively contaminated for which the licensee foresees no further use, and (ii) transfers this material or component to a licensed land disposal facility or to a licensed waste collector or processor for handling or treatment prior to disposal. A licensee performing processing or decontamination services may be a "waste generator" if the transfer of low-level radioactive waste from its facility is defined as "residual waste."
"Waste handling licensees" mean persons licensed to receive and store radioactive wastes prior to disposal or persons licensed to dispose of radioactive waste.
"Waste processor" means an entity, operating under a specific license, whose principal purpose is to process, repackage, or otherwise treat low-level radioactive material or waste generated by others prior to eventual transfer of waste to a licensed low-level radioactive waste land disposal facility.
"Waste type" means a waste within a disposal container having a unique physical description (i.e., a specific waste descriptor code or description; or a waste sorbed on or solidified in a specifically defined media).
"Wedge filter" means a filter that effects continuous change in transmission over all or a part of the useful beam.
"Week" means seven consecutive days starting on Sunday.
"Weighting factor" or "wT" for an organ or tissue (T) means the proportion of the risk of stochastic effects resulting from irradiation of that organ or tissue to the total risk of stochastic effects when the whole body is irradiated uniformly. For calculatingthe effective dose equivalent, the values of wT are:
Organ Dose Weighting Factors |
Organ or Tissue | wT |
Gonads | 0.25 |
Breast | 0.15 |
Red bone marrow | 0.12 |
Lung | 0.12 |
Thyroid | 0.03 |
Bone surfaces | 0.03 |
Remainder | 0.30a/ |
Whole Body | 1.00b/ |
a/0.30 results from 0.06 for each of five "remainder" organs, excluding the skin and the lens of the eye, that receive the highest doses. |
b/For the purpose of weighting the external whole body dose for adding it to the internal dose, a single weighting factor, wT = 1.0, has been specified. The use of other weighting factors for external exposure will be approved on a case-by-case basis until such time as specific guidance is issued. |
"Well-bore" means a drilled hole in which wireline service operations or subsurface tracer studies are performed.
"Well-logging" means all operations involving the lowering and raising of measuring devices or tools that may contain sources of radiation into well-bores or cavities for the purpose of obtaining information about the well or adjacent formations.
"Whole body" means, for purposes of external exposure, head, trunk including male gonads, arms above the elbow, or legs above the knee.
"Wireline" means a cable containing one or more electrical conductors that is used to lower and raise logging tools in the well-bore.
"Wireline service operation" means any evaluation or mechanical service that is performed in the well-bore using devices on a wireline.
"Worker" means an individual engaged in work under a license or registration issued by the agency and controlled by a licensee or registrant but does not include the licensee or registrant.
"Working level" or "WL" means any combination of short-lived radon daughters in one liter of air that will result in the ultimate emission of 1.3E+5 MeV of potential alpha particle energy. The short-lived radon daughters of radon-222 are polonium-218, lead-214, bismuth-214, and polonium-214; and those of radon-220 are polonium-216, lead-212, bismuth-212, and polonium-212.
"Working level month" or "WLM" means an exposure to one working level for 170 hours. Two thousand working hours per year divided by 12 months per year is approximately equal to 170 hours per month.
"Written directive" means an order in writing for a specific patient, dated and signed by an authorized user prior to the administration of a radiopharmaceutical or radiation, except as specified in subdivision 6 of this definition, containing the following information:
1. For any administration of quantities greater than 1.11 megabecquerels (30 mCi) of sodium iodide I-125 or I-131: the radionuclide, and dosage;
2. For a therapeutic administration of a radiopharmaceutical other than sodium iodide I-125 or I-131: the radiopharmaceutical, dosage, and route of administration;
3. For gamma stereotactic radiosurgery: target coordinates, collimator size, plug pattern, and total dose;
4. For teletherapy: the total dose, dose per fraction, treatment site, and overall treatment period;
5. For high-dose-rate remote afterloading brachytherapy: the radionuclide, treatment site, and total dose; or
6. For all other brachytherapy,
a. Prior to implantation: the radionuclide, number of sources, and source strengths; and
b. After implantation but prior to completion of the procedure: the radionuclide, treatment site, and total source strength and exposure time (or, equivalently, the total dose).
"X-ray control" means a device that controls input power to the x-ray high-voltage generator or the x-ray tube. It includes equipment such as timers, phototimers, automatic brightness stabilizers, and similar devices, which control the technique factors of an x-ray exposure.
"X-ray exposure control" means a device, switch, button or other similar means by which an operator initiates or terminates the radiation exposure. The x-ray exposure control may include such associated equipment as timers and back-up timers.
"X-ray equipment" means an x-ray system, subsystem, or component thereof. Types of x-ray equipment are as follows:
1. "Mobile x-ray equipment" means x-ray equipment mounted on a permanent base with wheels or casters for moving while completely assembled.
2. "Portable x-ray equipment" means x-ray equipment designed to be hand-carried.
3. "Stationary x-ray equipment" means x-ray equipment that is installed in a fixed location.
"X-ray field" means that area of the intersection of the useful beam and any one of the sets of planes parallel to and including the plane of the image receptor, whose perimeter is the locus of points at which the AKR is one-fourth of the maximum in the intersection.
"X-ray high-voltage generator" means a device that transforms electrical energy from the potential supplied by the x-ray control to the tube operating potential. The device may also include means for transforming alternating current to direct current, filament transformers for the x-ray tubes, high-voltage switches, electrical protective devices, and other appropriate elements.
"X-ray system" means an assemblage of components for the controlled production of x-rays. It includes minimally an x-ray high-voltage generator, an x-ray control, a tube housing assembly, a beam-limiting device, and the necessary supporting structures. Additional components that function with the system are considered integral parts of the system.
"X-ray table" means a patient support device with its patient support structure (tabletop) interposed between the patient and the image receptor during radiography or fluoroscopy. This includes, but is not limited to, any stretcher equipped with a radiolucent panel and any table equipped with a cassette tray (or bucky), cassette tunnel, fluoroscopic image receptor, or spot-film device beneath the tabletop.
"X-ray tube" means any electron tube that is designed for the conversion of electrical energy into x-ray energy.
"Year" means the period of time beginning in January used to determine compliance with the provisions of this chapter. The licensee or registrant may change the starting date of the year used to determine compliance by the licensee or registrant provided that the change is made at the beginning of the year. If a licensee or registrant changes in a year, the licensee or registrant shall assure that no day is omitted or duplicated in consecutive years.
12VAC5-481-421. Requirements for license to initially transfer source material for use under the small quantities of source material general license.
A. An application for a specific license to initially transfer source material for use under 12VAC5-481-420 A or equivalent regulations of the NRC or another agreement state will be approved if:
1. The applicant satisfies the general requirements specified in 12VAC5-481-450; and
2. The applicant submits adequate information on, and the agency approves the methods to be used for quality control, labeling, and providing safety instructions to recipients.
B. Conditions of licenses to initially transfer source material for use under the small quantities of source material general license: quality control, labeling, safety instructions, and records and reports.
1. Each person licensed under subsection A of this section shall label the immediate container of each quantity of source material with the type of source material and quantity of material and the words, "radioactive material."
2. Each person licensed under subsection A of this section shall ensure that the quantities and concentrations of source material are as labeled and indicated in any transfer records.
3. Each person licensed under subsection A of this section shall provide the information specified in this subdivision to each person to whom source material is transferred for use under 12VAC5-481-420 A or equivalent provisions of the NRC or another agreement state. This information shall be transferred before the source material is transferred for the first time in each calendar year to the particular recipient. The required information includes:
a. A copy of 12VAC5-481-420 A and 12VAC5-481-570, or relevant equivalent regulations of the NRC or another agreement state.
b. Appropriate radiation safety precautions and instructions relating to handling, use, storage, and disposal of the material.
4. Each person licensed under subsection A of this section shall report transfers as follows:
a. File a report with the Director, Office of Federal and State Materials and Environmental Management Programs Nuclear Material Safety and Safeguards, U.S. Nuclear Regulatory Commission, Washington, DC 20555. The report shall include the following information:
(1) The name, address, and license number of the person who transferred the source material;
(2) For each general licensee under 10 CFR 40.22 or equivalent agreement state provisions to whom greater than 50 grams (0.11 lb) of source material has been transferred in a single calendar quarter, the name and address of the general licensee to whom source material is distributed; a responsible agent, by name, position, or both and phone number, of the general licensee to whom the material was sent and the type, physical form, and quantity of source material transferred; and
(3) The total quantity of each type and physical form of source material transferred in the reporting period to all such generally licensed recipients.
b. File a report with the agency and other agreement state agencies that identifies all persons operating under provisions equivalent to 12VAC5-481-420 A to whom greater than 50 grams (0.11 lb) of source material has been transferred within a single calendar quarter. The report shall include the following information specific to those transfers made to the agreement state to which the report is being made:
(1) The name, address, and license number of the person who transferred the source material;
(2) The name and address of the general licensee to whom source material was distributed; a responsible agent, by name, position, or both and phone number, of the general licensee to whom the material was sent; and the type, physical form, and quantity of source material transferred; and
(3) The total quantity of each type and physical form of source material transferred in the reporting period to all such generally licensed recipients within the agreement state.
c. Submit each report by January 31 of each year covering all transfers for the previous calendar year. If no transfers were made to persons generally licensed under 12VAC5-481-420 A or equivalent NRC and other agreement state provisions during the current period, a report shall be submitted to the agency indicating so. If no transfers have been made to general licensees of the NRC or in a particular agreement state during the reporting period, this information shall be reported to the NRC or responsible agreement state agency upon request of the agency.
5. Each person licensed under subsection A of this section shall maintain all information that supports the reports required by this section concerning each transfer to a general licensee for a period of one year after the event is included in a report to the agency, the NRC, or another agreement state.
12VAC5-481-451. Physical protection of Category 1 and Category 2 quantities of radioactive material.
A. Any licensee who possesses or uses an aggregated quantity of Category 1 or Category 2 radioactive material equal to or in excess of those in subdivision 1 of this subsection shall establish a physical protection program that meets all requirements detailed in this section.
1. Radionuclides of concern.
Radionuclide | Category 1 (TBq)1,2 | Category 1 (Ci)1,2 | Category 2 (TBq)1,2 | Category 2 (Ci)1,2 |
Am-241 | 60 | 1,620 | 0.6 | 16.2 |
Am-241/Be | 60 | 1,620 | 0.6 | 16.2 |
Cf-252 | 20 | 540 | 0.2 | 5.4 |
Cm-244 | 50 | 1,350 | 0.5 | 13.5 |
Co-60 | 30 | 810 | 0.3 | 8.1 |
Cs-137 | 100 | 2,700 | 1 | 27 |
Gd-153 | 1,000 | 27,000 | 10 | 270 |
Ir-192 | 80 | 2,160 | 0.8 | 21.6 |
Pm-147 | 40,000 | 1,080,000 | 400 | 10,800 |
Pu-238 | 60 | 1,620 | 0.6 | 16.2 |
Pu-239/Be | 60 | 1,620 | 0.6 | 16.2 |
Ra-226 | 40 | 1,080 | 0.4 | 10.8 |
Se-75 | 200 | 5,400 | 2 | 54 |
Sr-90 (Y-90) | 1,000 | 27,000 | 10 | 270 |
Tm-170 | 20,000 | 540,000 | 200 | 5,400 |
Yb-169 | 300 | 8,100 | 3 | 81 |
Combinations of radioactive materials listed above3 | | | See footnote 4 below | |
1The aggregate activity of multiple, collocated sources of the same radionuclides should be included when the total activity equals or exceeds the Category 1 or Category 2 threshold. 2The primary values used for compliance are TBq. The curie (Ci) values are rounded to two significant figures for informational purposes only. 3Radioactive materials are to be considered aggregated or collocated if breaching a common physical barrier (e.g., a locked door at the entrance to a storage room) would allow access to the radioactive material or devices containing the radioactive material. 4If several radionuclides are aggregated, the sum of the ratios of the activity of each source, i of radionuclide, n, A (i,n), to the Category 1 or Category 2 threshold for radionuclide n, Qn, listed for that radionuclide equals or exceeds one. [(aggregated source activity for radionuclide A) / (quantities of concern for radionuclide A)] + [(aggregated source activity for radionuclide B) / (quantities of concern for radionuclide B)] + etc…. ≥ 1. |
2. A licensee that possesses radioactive waste that contains Category 1 or Category 2 quantities of radioactive material is exempt from the requirements of this section.
3. A licensee that possesses radioactive waste that contains discrete sources, ion-exchange resins, or activated material that weighs less than 2,000 kg (4,409 lbs) is not exempt from the requirements of this section. The licensee shall implement the following requirements to secure the radioactive waste:
a. Use continuous physical barriers that allow access to the radioactive waste only through established access control points;
b. Use a locked door or gate with monitored alarm at the access control point;
c. Assess and respond to each actual or attempted unauthorized access to determine whether an actual or attempted theft, sabotage, or diversion occurred; and
d. Immediately notify the local law-enforcement agency (LLEA) and request an armed response from the LLEA upon determination that there was an actual or attempted theft, sabotage, or diversion of the radioactive waste.
B. Background investigations and access authorization program.
1. Personnel access authorization requirements for Category 1 or Category 2 quantities of radioactive material.
a. Each licensee that possesses an aggregated quantity of radioactive material that equals or exceeds the Category 2 threshold shall establish, implement, and maintain its access authorization program in accordance with the requirements in this subsection. An applicant for a new license and each licensee that would become newly subject to the requirements in this subsection upon an amendment request of its license shall implement the requirements of this subsection, as appropriate, before taking possession of an aggregated quantity of radioactive material that equals or exceeds the Category 2 threshold. Any licensee that has not previously implemented the increased control requirements of this section shall implement the provisions of this subsection before aggregating radioactive material to a quantity that equals or exceeds the Category 2 threshold.
b. The licensee's access authorization program shall ensure that the individuals specified in subdivision 1 c of this subsection are trustworthy and reliable.
c. Licensees shall subject the following individuals to an access authorization program:
(1) Any individual whose assigned duties require unescorted access to Category 1 or Category 2 quantities of radioactive material; and
(2) Reviewing officials.
d. Licensees shall approve for unescorted access to Category 1 or Category 2 quantities of radioactive material only those individuals whose assigned job duties require unescorted access to Category 1 or Category 2 quantities of radioactive material.
e. Licensees need not subject the categories of individuals listed in subdivision 5 a of this subsection to the investigation elements of the access authorization program.
2. Access authorization program requirements.
a. Granting unescorted access authorization.
(1) Licensees shall implement the requirements of this subsection for granting initial or reinstated unescorted access authorization.
(2) Individuals who have been determined to be trustworthy and reliable shall also complete the security training required by subdivision C 2 c of this section before being allowed unescorted access to Category 1 or Category 2 quantities of radioactive material.
b. Reviewing officials.
(1) Reviewing officials are the only individuals who may make trustworthiness and reliability determinations that allow individuals to have unescorted access to Category 1 or Category 2 quantities of radioactive materials possessed by the licensee.
(2) Each licensee shall name one or more individuals to be reviewing officials. After completing the background investigation on the reviewing official, the licensee shall provide under oath or affirmation a certification that the reviewing official is deemed trustworthy and reliable by the licensee. The fingerprints of the named reviewing official shall be taken by a law-enforcement agency, a federal or state agency that provides fingerprinting services to the public, or a commercial fingerprinting service authorized by a state to take fingerprints. The licensee shall recertify that the reviewing official is deemed trustworthy and reliable every 10 years in accordance with subdivision 3 c of this subsection.
(3) Reviewing officials shall be permitted to have unescorted access to Category 1 or Category 2 quantities of radioactive material.
(4) Reviewing officials cannot approve other individuals to act as reviewing officials.
(5) A reviewing official does not need to undergo a new background investigation before being named by the licensee as the reviewing official if:
(a) The individual has undergone a background investigation that included fingerprinting and an FBI criminal history records check and has been determined to be trustworthy and reliable by the licensee; or
(b) The individual is subject to a category listed in subdivision 5 a of this subsection.
c. Informed consent.
(1) Licensees may not initiate a background investigation without the informed and signed consent of the subject individual. This consent shall include authorization to share personal information with other individuals or organizations as necessary to complete the background investigation. Before a final adverse determination, the licensee shall provide the individual with an opportunity to correct any inaccurate or incomplete information that is developed during the background investigation. Licensees do not need to obtain signed consent from those individuals who meet the requirements of subdivision 3 b of this subsection. A signed consent shall be obtained prior to any reinvestigation.
(2) The subject individual may withdraw his consent at any time. Licensees shall inform the individual that:
(a) If an individual withdraws his consent, the licensee may not initiate elements of the background investigation that were not in progress at the time the individual withdrew his consent; and
(b) The withdrawal of consent for the background investigation is sufficient cause of denial or termination of unescorted access authorization.
d. Any individual who is applying for unescorted access authorization shall disclose the personal history information that is required by the licensee's access authorization program for the reviewing official to make a determination of the individual's trustworthiness and reliability. Refusal to provide, or the falsification of, any personal history information required by this subsection is sufficient cause for denial or termination of unescorted access.
e. Determination basis.
(1) The reviewing official shall determine whether to permit, deny, unfavorably terminate, maintain, or administratively withdraw an individual's unescorted access authorization based on an evaluation of all the information collected to meet the requirements of this subsection.
(2) The reviewing official may not permit any individual to have unescorted access until the reviewing official has evaluated all the information collected to meet the requirements of this subsection and determined that the individual is trustworthy and reliable. The reviewing official may deny unescorted access to any individual based on information obtained at any time during the background investigation.
(3) The licensee shall document the basis for concluding whether or not there is reasonable assurance that an individual is trustworthy and reliable.
(4) The reviewing official may terminate or administratively withdraw an individual's unescorted access authorization based on information obtained after the background investigation has been completed and the individual granted unescorted access information.
(5) Licensees shall maintain a list of persons currently approved for unescorted access authorization. When a licensee determines that a person no longer requires unescorted access or meets the access authorization requirement, the licensee shall remove the person from the approved list as soon as possible, but no later than seven working days, and take prompt measures to ensure that the individual is unable to have unescorted access to the material.
f. Licensees shall develop, implement, and maintain written procedures for implementing the access authorization program. The procedures shall include the provisions for the notification of individuals who are denied unescorted access. The procedures shall include provisions for the review, at the request of the affected individual, of a denial or termination of unescorted access authorization. The procedures shall contain a provision to ensure that the individual is informed of the grounds for the denial or termination of unescorted access authorization and allow the individual an opportunity to provide additional relevant information.
g. Right to correct and complete information.
(1) Prior to any final adverse determination, licensees shall provide each individual subject to this subsection with the right to complete, correct, and explain information obtained as a result of the licensee's background investigation. Confirmation of receipt by the individual of this notification shall be maintained by the licensee for a period of one year from the date of the notification.
(2) If, after reviewing his criminal history record, an individual believes that it is incorrect or incomplete in any respect and wishes to change, correct, update, or explain anything in the record, the individual may initiate challenge procedures. These procedures include direct application by the individual challenging the record to the law-enforcement agency that contributed the questioned information or a direct challenge as to the accuracy or completeness of any entry on the criminal history record to the Federal Bureau of Investigation, Criminal Justice Information Services (CJIS) Division, ATTN: SCU, Mod. D-2, 1000 Custer Hollow Road, Clarksburg, WV 26306 as set forth in 28 CFR 16.30 through 28 CFR 16.34. In the latter case, the Federal Bureau of Investigation (FBI) will forward the challenge to the agency that submitted the data and will request that the agency verify or correct the challenged entry. Upon receipt of an official communication directly from the agency that contributed the original information, the FBI Identification Division will make any change necessary in accordance with the information supplied by that agency. Licensees shall provide at least 10 days for an individual to initiate action to challenge the results of an FBI criminal history records check after the record being made available for his review. The licensee may make a final adverse determination based upon the criminal history records only after receipt of the FBI's confirmation or correction of the record.
h. Records.
(1) The licensee shall retain documentation regarding the trustworthiness and reliability of individual employees for three years from the date the individual no longer requires unescorted access to Category 1 or Category 2 quantities of radioactive material.
(2) The licensee shall retain a copy of the current access authorization program procedures as a record for three years after the procedure is no longer needed. If any portion of the procedure is superseded, the licensee shall retain the superseded material for three years after the record is superseded.
(3) The licensee shall retain the list of individuals approved for unescorted access authorization for three years after the list is superseded or replaced.
3. Background investigations.
a. Before allowing an individual unescorted access to Category 1 or Category 2 quantities of radioactive material or to the devices containing the material, licensees shall complete a background investigation of the individual seeking unescorted access authorization. The scope of the investigation shall encompass at least the seven years preceding the date of the background investigation or since the individual's 18th birthday, whichever is shorter. The background investigation shall include at a minimum:
(1) Fingerprinting and an FBI identification and criminal history records check in accordance with subdivision 4 of this subsection;
(2) Verification of true identity of the individual who is applying for unescorted access authorization. A licensee shall review official identification documents (e.g., driver's license; passport; government identification; certificate of birth issued by the state, province, or country of birth) and compare the documents to personal information data provided by the individual to identify any discrepancy in the information. Licensees shall document the type, expiration, and identification number of the identification document or maintain a photocopy of identifying documents on file in accordance with subdivision 6 of this subsection. Licensees shall certify in writing that the identification was properly reviewed and shall maintain the certification and all related documents for review upon inspection;
(3) Verification of employment history, including military history. Licensees shall verify the individual's employment with each previous employer for the most recent seven years before the date of application;
(4) Verification that the individual participated in the education process during the claimed period;
(5) Completion of reference checks to determine the character and reputation of the individual who has applied for unescorted access authorization. Unless other references are not available, reference checks may not be conducted with any person who is known to be a close member of the individual's family, including but not limited to, the individual's spouse, parents, siblings, or children, or any individual who resides in the individual's permanent household. Reference checks under this subsection shall be limited to whether the individual has been and continues to be trustworthy and reliable;
(6) To the extent possible, obtain independent information to corroborate the information provided by the individual (e.g., seek references not supplied by the individual); and
(7) If a previous employer, educational institution, or any other entity with which the individual claims to have been engaged fails to provide the information or indicates an inability or unwillingness to provide information within a timeframe deemed appropriate by the licensee but at least after 10 business days of the request or if the licensee is unable to reach the entity, the licensee shall document the refusal, unwillingness, or inability in the record of investigation and attempt to obtain the information from an alternate source.
b. Individuals who have been determined to be trustworthy and reliable for unescorted access to Category 1 or Category 2 quantities of radioactive material in accordance with 12VAC5-481-451, "Increased controls and fingerprinting," as effective on October 3, 2008, can continue to have unescorted access to Category 1 and Category 2 quantities of radioactive material without further investigation. These individuals shall be subject to the reinvestigation requirement of subdivision 3 c of this subsection.
c. Licensees shall conduct a reinvestigation every 10 years for any individual with unescorted access to Category 1 or Category 2 quantities of radioactive material. The reinvestigation shall consist of fingerprinting and an FBI identification and criminal history records check in accordance with subdivision 4 of this subsection. The reinvestigations shall be completed within 10 years of the date on which these elements were last completed.
4. Requirements for criminal history records checks of individuals granted unescorted access to Category 1 or Category 2 quantities of radioactive material.
a. General performance objective and requirements.
(1) Except for those individuals listed in subdivision 5 a of this subsection and those individuals grandfathered under subdivision 3 b of this subsection, each licensee subject to the provisions of this section shall fingerprint each individual who is to be permitted unescorted access to Category 1 or Category 2 quantities of radioactive material. The licensee shall submit all collected fingerprints to the NRC for transmission to the FBI. The licensee shall use the information received from the FBI as part of the required background investigation to determine whether to grant or deny further unescorted access to Category 1 or Category 2 quantities of radioactive materials for that individual.
(2) The licensee shall notify each affected individual that his fingerprints will be used to secure a review of his criminal history record and shall inform him of the procedures for revising the record or adding explanations to the record.
(3) Fingerprinting is not required if a licensee is reinstating an individual's unescorted access authorization to Category 1 or Category 2 quantities of radioactive material if:
(a) The individual returns to the same facility that granted unescorted access authorization within 365 days of the termination of his unescorted access authorization; and
(b) The previous access was terminated under favorable conditions.
(4) Fingerprints do not need to be taken if an individual who is an employee of a licensee, contractor, manufacturer, or supplier has been granted unescorted access to Category 1 or Category 2 quantities of radioactive material, access to safeguards information, or safeguards information-modified handling by another licensee based upon a background investigation conducted under this subsection, regulations or Fingerprint Orders from another agreement state, or 10 CFR Part 73. An existing criminal history records check file may be transferred to the licensee asked to grant unescorted access in accordance with the provisions of subdivision 6 c of this subsection.
(5) Licensees shall use the information obtained as part of a criminal history records check solely for the purpose of determining an individual's suitability for unescorted access authorization to Category 1 or Category 2 quantities of radioactive materials, access to safeguards information, or safeguards information-modified handling.
b. Prohibitions.
(1) Licensees may not base a final determination to deny an individual unescorted access authorization to Category 1 or Category 2 quantities of radioactive material solely on the basis of information received from the FBI involving:
(a) An arrest more than one year old for which there is no information of the disposition of the case; or
(b) An arrest that resulted in dismissal of the charge or an acquittal.
(2) Licensees may not use information received from a criminal history records check obtained under this subsection in a manner that would infringe upon the rights of any individual under the First Amendment to the Constitution of the United States, nor shall licensees use the information in any way that would discriminate among individuals on the basis of race, religion, national origin, gender, or age.
c. Procedures for processing of fingerprint checks.
(1) For the purpose of complying with this subsection, licensees shall submit to the U.S. Nuclear Regulatory Commission, Director, Division of Facilities and Security, 11545 Rockville Pike, ATTN: Criminal History Program/Mail Stop T-03B46M TWB-05 B32M, Rockville, MD, 20852-2738 20852, one completed, legible standard fingerprint card (form FD-258, ORIMDNRCOOOZ), electronic fingerprint scan, or, where practicable, other fingerprint record for each individual requiring unescorted access to Category 1 or Category 2 quantities of radioactive material. Copies of these forms may be obtained by writing the Office of Chief Information Services Officer, U.S. Nuclear Regulatory Commission, Washington, DC 20555-0001, by calling (630) 829-9565, or by email to forms.resource@nrc.gov. Guidance on submitting electronic fingerprints can be found at http://www.nrc.gov/site-help/e-submittals.html.
(2) Fees for processing of fingerprint cards are due upon application. Licensees shall submit payment with the application for the processing of fingerprints through corporate check, certified check, cashier's check, money order, or electronic payment, made payable to the "U.S. NRC." (For guidance on making electronic payments, contact the Security Branch, Division of Facilities and Security at (301) 492-3531.) Combined payment for multiple applications is acceptable. The NRC publishes the amount of the fingerprint check application fee on the NRC public website. To find the current fee amount, go to the Electronic Submittals page at http://www.nrc.gov/site-help/e-submittals.html and see the link for the Criminal History Program under Electronic Submission Systems.
(3) The NRC will forward to the submitting licensee all data received from the FBI as a result of the licensee's application for a criminal history records check.
5. Relief.
a. Fingerprinting, identification and criminal history records checks, and other elements of the background investigation required by this subsection are not required for the following individuals prior to granting unescorted access to Category 1 or Category 2 quantities of radioactive material:
(1) An employee of the NRC or of the executive branch of the U.S. government who has undergone fingerprinting for a prior U.S. government criminal history records check;
(2) A member of Congress;
(3) An employee of a member of Congress or congressional committee who has undergone fingerprinting for a prior U.S. government criminal history records check;
(4) The governor of a state or his designated state employee representative;
(5) Federal, state, or local law-enforcement personnel;
(6) State radiation control program directors and state homeland security advisors or their designated employee representatives;
(7) State radiation program employees conducting security inspections on behalf of the NRC under an agreement executed under § 274i of the Atomic Energy Act (42 USC § 2021i);
(8) Representatives of the International Atomic Energy Agency (IAEA) engaged in activities associated with the U.S./IAEA Safeguards Agreement who have been certified by the NRC;
(9) Emergency response personnel who are responding to an emergency;
(10) Commercial vehicle drivers for road shipments of Category 1 and Category 2 quantities of radioactive material;
(11) Package handlers at transportation facilities such as freight terminals and railroad yards;
(12) Any individual who has an active federal security clearance and provides the appropriate documentation. Written confirmation from the agency or employer that granted the federal security clearance or reviewed the criminal history records check shall be provided to the licensee. The licensee shall retain this documentation for a period of three years from the date the individual no longer requires unescorted access to Category 1 or Category 2 quantities of radioactive material; and
(13) Any individual employed by a service provider licensee for whom the service provider licensee has conducted the background investigation for the individual and approved the individual for unescorted access to Category 1 or Category 2 quantities of radioactive material. Written verification from the service provider shall be provided to the licensee. The licensee shall retain the documentation for a period of three years from the date the individual no longer requires unescorted access to Category 1 or Category 2 quantities of radioactive material.
b. Fingerprinting and identification and criminal history records checks required by this subsection are not required for an individual who has had a favorably adjudicated U.S. Government criminal history records check within the last five years, under a comparable U.S. Government program involving fingerprinting and an FBI identification and criminal history records check, and the individual provides the appropriate documentation. Written confirmation from the agency or employer that reviewed the criminal history records check shall be provided to the licensee. The licensee shall retain this documentation for a period of three years from the date the individual no longer requires unescorted access to Category 1 or Category 2 quantities of radioactive material. These programs include, but are not limited to:
(1) National Agency Check;
(2) Transportation Worker Identification Credentials (TWIC) under 49 CFR Part 1572;
(3) Bureau of Alcohol, Tobacco, Firearms, and Explosives background check and clearances under 27 CFR Part 555;
(4) Health and Human Services security risk assessments for possession and use of select agents and toxins under 42 CFR Part 73;
(5) Hazardous material security threat assessment for hazardous material endorsement to commercial driver's license under 49 CFR Part 1572; and
(6) Customs and Border Protection's Free and Secure Trade (FAST) Program.
6. Protection of information.
a. Each licensee that obtains background information on an individual under this subsection shall establish and maintain a system of files and written procedures for protection of the record and the personal information from unauthorized disclosure.
b. The licensee may not disclose the record or personal information collected and maintained to persons other than the subject individual, his representative, or to those who have a need to have access to the information in performing assigned duties in the process of granting or denying unescorted access to Category 1 or Category 2 quantities of radioactive material. No individual authorized to have access to the information may disseminate the information to any other individual who does not have a need to know.
c. The personal information obtained on an individual from a background investigation may be provided to another licensee:
(1) Upon the individual's written request to the licensee holding the data to disseminate the information contained in that individual's file; and
(2) The recipient licensee verifies information such as name, date of birth, social security number, gender, and other applicable physical characteristics.
d. The licensee shall make background investigation records obtained under this subsection available for examination by an authorized representative of the agency to determine compliance with the regulations and laws.
e. The licensee shall retain all fingerprint and criminal history records (including data indicating no record) received from the FBI, or a copy of these records if the individual's file has been transferred, on an individual for three years from the date the individual no longer requires unescorted access to Category 1 or Category 2 quantities of radioactive material.
7. Access authorization program review.
a. Each licensee shall be responsible for the continuing effectiveness of the access authorization program. Each licensee shall ensure that access authorization programs are reviewed to confirm compliance with the requirements of this subsection and that comprehensive actions are taken to correct any noncompliance that is identified. The review program shall evaluate all program performance objectives and requirements. The review shall be performed at least annually.
b. The results of the reviews, along with all recommendations, shall be documented. Each review report shall identify conditions that are adverse to the proper performance of the access authorization program; the cause of the conditions and, when appropriate, recommend corrective actions; and corrective actions taken. The licensee shall review the findings and take additional corrective actions necessary to preclude repetition of the condition, including reassessment of the deficient areas where indicated.
c. Review records shall be maintained for three years.
C. Physical protection requirements during use.
1. Security program.
a. Each licensee that possesses an aggregated Category 1 or Category 2 quantity of radioactive material shall establish, implement, and maintain a security program in accordance with the requirements of this subsection. An applicant for a new license and each licensee that would become newly subject to the requirements of this subsection upon an amendment request for modification of its license shall implement the requirements of this subsection, as appropriate, before taking possession of an aggregated Category 1 or Category 2 quantity of radioactive material. Any licensee that has not previously implemented the requirements of this subsection shall provide written notification to the agency at least 90 days before aggregating radioactive material to a quantity that equals or exceeds the Category 2 threshold.
b. Each licensee shall establish, implement, and maintain a security program that is designed to monitor and, without delay, detect, assess, and respond to an actual or attempted unauthorized access to Category 1 or Category 2 quantities of radioactive material.
c. Each licensee's security program shall include the program features, as appropriate, described in subdivisions 2 through 8 of this subsection.
2. General security program requirements.
a. Security plan.
(1) Each licensee identified in subdivision 1 a of this subsection shall develop a written security plan specific to its facilities and operations. The purpose of the security plan is to establish the licensee's overall security strategy to ensure the integrated and effective functioning of the security program required by this subsection. The security plan shall, at a minimum, (i) describe the measures and strategies used to implement the requirements of this subsection and (ii) identify the security resources, equipment, and technology used to satisfy the requirements of this subsection.
(2) The security plan shall be reviewed and approved by the individual with overall responsibility for the security program.
(3) A licensee shall revise its security plan as necessary to ensure the effective implementation of agency requirements. The licensee shall ensure that (i) the revision has been reviewed and approved by the individual with overall responsibility for the security program and (ii) the affected individuals are instructed on the revised plan before the changes are implemented.
(4) The licensee shall retain a copy of the current security plan as a record for three years after the security plan is no longer required. If any portion of the plan is superseded, the licensee shall retain the superseded material for three years after the record is superseded.
b. Implementing procedures.
(1) The licensee shall develop and maintain written procedures that document how the requirements of this subsection and the security plan will be met.
(2) The implementing procedures and revisions to these procedures shall be approved in writing by the individual with overall responsibility for the security program.
(3) The licensee shall retain a copy of the current procedure as a record for three years after the procedure is no longer needed. Superseded portions of the procedure shall be retained for three years after the record is superseded.
c. Training.
(1) Each licensee shall conduct training to ensure that those individuals implementing the security program possess and maintain the knowledge, skills, and abilities to carry out their assigned duties and responsibilities effectively. The training shall include at a minimum, instruction on:
(a) The licensee's security program and procedures to secure Category 1 or Category 2 quantities of radioactive material, and the purpose and function of the security measures employed;
(b) The responsibility to report promptly to the licensee any condition that causes or may cause a violation of agency requirements;
(c) The responsibility of the licensee to report promptly to the local law-enforcement agency and the agency any actual or attempted theft, sabotage, or diversion of Category 1 or Category 2 quantities of radioactive material; and
(d) The appropriate response to security alarms.
(2) In determining those individuals who shall be trained on the security program, the licensee shall consider each individual's assigned activities during authorized use and response to potential situations involving actual or attempted theft, diversion, or sabotage of Category 1 or Category 2 quantities of radioactive material. The extent of the training shall be commensurate with the individual's potential involvement in the security of Category 1 or Category 2 quantities of radioactive material.
(3) Refresher training shall be provided at a frequency not to exceed 12 months and when significant changes have been made to the security program. This training shall include (i) review of the training requirements of this subsection and changes made to the security program since the last training; (ii) reports on all relevant security issues, problems, and lessons learned; (iii) relevant results of agency inspections; and (iv) relevant results of the licensee's program review and testing and maintenance.
(4) The licensee shall maintain records of the initial and refresher training for three years from the date of the training. The training records shall include dates of the training, topics covered, a list of licensee personnel in attendance, and related information.
d. Protection of information.
(1) Licensees authorized to possess Category 1 or Category 2 quantities of radioactive material shall limit access to and prevent the unauthorized disclosure of their security plan, implementing procedures, and the list of individuals who have been approved for unescorted access.
(2) Efforts to limit access shall include the development, implementation, and maintenance of written policies and procedures for controlling access to and for proper handling and protection against unauthorized disclosure of the security plan and implementing procedures.
(3) Before granting an individual access to the security plan or implementing procedures, licensees shall:
(a) Evaluate an individual's need to know the security plan or implementing procedures; and
(b) If the individual has not been authorized for unescorted access to Category 1 or Category 2 quantities of radioactive material, the licensee shall complete a background investigation to determine the individual's trustworthiness and reliability. A trustworthiness and reliability determination shall be conducted by the reviewing official and shall include the background investigation elements contained in subdivisions B 3 a (2) through (7) of this section.
(4) Licensees need not subject any individual to background investigation elements for protection of information if that individual is included in the categories of individuals listed in subdivisions B 5 a (1) through (12) of this section or is a security service provider employee, provided written verification that the employee has been determined to be trustworthy and reliable, by the required background investigation in subdivisions B 3 a (2) though (7) of this subsection, has been provided by the security service provider.
(5) The licensee shall document the basis for concluding that an individual is trustworthy and reliable and should be granted access to the security plan or implementing procedures.
(6) Licensees shall maintain a list of persons currently approved for access to the security plan or implementing procedures. When a licensee determines that a person no longer needs access to the security plan or implementing procedures or no longer meets the access authorization requirements for access to the information, the licensee shall remove the person from the approved list as soon as possible, but no later than seven working days after the determination, and take prompt measures to ensure that the individual is unable to obtain the security plan or implementing procedures.
(7) When not in use, the licensee shall store its security plan and implementing procedures in a manner to prevent unauthorized access. Information stored in nonremovable electronic form shall be password protected.
(8) The licensee shall retain as a record a copy of the information protection procedures and the list of individuals approved for access to the security plan or implementing procedures for three years after the document has been superseded.
3. Local law-enforcement agency (LLEA) coordination.
a. A licensee subject to this subsection shall coordinate, to the extent practicable, with an LLEA for responding to threats to the licensee's facility, including any necessary armed response. The information provided to the LLEA shall include:
(1) A description of the facilities and the Category 1 and Category 2 quantities of radioactive materials along with a description of the licensee's security measures that have been implemented to comply with this subsection; and
(2) A notification that the licensee will request a timely armed response by the LLEA to any actual or attempted theft, sabotage, or diversion of Category 1 or Category 2 quantities of material.
b. The licensee shall notify the agency within three business days if:
(1) The LLEA has not responded to the request for coordination within 60 days of the coordination request; or
(2) The LLEA notifies the licensee that the LLEA does not plan to participate in coordination activities.
c. The license shall document its efforts to coordinate with the LLEA. The documentation shall be kept for three years.
d. The licensee shall coordinate with the LLEA at least every 12 months, or when changes to the facility design or operation adversely affect the potential vulnerability of the licensee's material to theft, sabotage, or diversion.
4. Security zones.
a. Licensees shall ensure that all aggregated Category 1 or Category 2 quantities of radioactive material are used or stored within licensee-established security zones. Security zones may be permanent or temporary.
b. Temporary security zones shall be established as necessary to meet the licensee's transitory or intermittent business activities, such as periods of maintenance, source delivery, and source replacement.
c. Security zones shall, at a minimum, allow unescorted access only to approved individuals by:
(1) Isolation of Category 1 and Category 2 quantities of radioactive materials by the use of continuous physical barriers that allow access to the security zone only through established access control points. A physical barrier is a natural or man-made structure or formation sufficient for the isolation of the Category 1 or Category 2 quantities of radioactive material within a security zone;
(2) Direct control of the security zone by approved individuals at all times; or
(3) A combination of continuous physical barriers and direct control.
d. For Category 1 quantities of radioactive material during periods of maintenance, source receipt, preparation for shipment, installation, or source removal or exchange, the licensee shall, at a minimum, provide sufficient individuals approved for unescorted access to maintain continuous surveillance of sources in temporary security zones and in any security zone in which physical barriers or intrusion detection systems have been disabled to allow such activities.
e. Individuals not approved for unescorted access to Category 1 or Category 2 quantities of radioactive material shall be escorted by an approved individual when in a security zone.
5. Monitoring, detection, and assessment.
a. Monitoring and detection.
(1) Licensees shall establish and maintain the capability to continuously monitor and detect without delay all unauthorized entries into its security zones. Licensees shall provide the means to maintain continuous monitoring and detection capability in the event of a loss of the primary power source, or provide for an alarm and response in the event of a loss of this capability to continuously monitor and detect unauthorized entries.
(2) Monitoring and detection shall be performed by:
(a) A monitored intrusion detection system that is linked to an onsite or offsite central monitoring facility;
(b) Electronic devices for intrusion detection alarms that will alert nearby facility personnel;
(c) A monitored video surveillance system;
(d) Direct visual surveillance by approved individuals located within the security zone; or
(e) Direct visual surveillance by a licensee designed individual located outside the security zone.
(3) A licensee subject to this subsection shall also have a means to detect unauthorized removal of the radioactive material from the security zone. This detection capability shall provide:
(a) For Category 1 quantities of radioactive material, immediate detection of any attempted unauthorized removal of the radioactive material from the security zone. Such immediate detection capability shall be provided by electronic sensors linked to an alarm, continuous monitored video surveillance, or direct visual surveillance; and
(b) For Category 2 quantities of radioactive material, weekly verification through physical checks, tamper indicating devices, use, or other means to ensure that the radioactive material is present.
b. Licensees shall immediately assess each actual or attempted unauthorized entry into the security zone to determine whether the unauthorized access was an actual or attempted theft, sabotage, or diversion.
c. For personnel and automated or electronic systems supporting the licensee's monitoring, detection, and assessments system, licensees shall:
(1) Maintain continuous capability for personnel communication and electronic data transmission and processing among site security systems; and
(2) Provide an alternate communication capability for personnel, and an alternative data transmission and processing capability, in the event of a loss of the primary means of communication or data transmission and processing. Alternative communications and data transmissions systems may not be subject to the same failure modes as the primary systems.
d. Licensees shall immediately respond to any actual or attempted unauthorized access to the security zones, or actual or attempted theft, sabotage, or diversion of Category 1 or Category 2 quantities of radioactive material at licensee facilities or temporary job sites. For any unauthorized access involving an actual or attempted theft, sabotage, or diversion of Category 1 or Category 2 quantities of radioactive material, the licensee's response shall include requesting, without delay, an armed response from the LLEA.
6. Maintenance and testing.
a. Each licensee subject to this subsection shall implement a maintenance and testing program to ensure that intrusion alarms, associated communication systems, and other physical components of the systems used to secure or detect unauthorized access to radioactive material are maintained in operable condition and are capable of performing their intended function when needed. The equipment relied on to meet the security requirements of this subsection shall be inspected and tested for operability and performance at the manufacturer's suggested frequency. If there is no frequency suggested by the manufacturer or the frequency specified is greater than three months, the testing shall be performed at least quarterly, not to exceed three months.
b. The licensee shall maintain records on the maintenance and testing activities for three years.
7. Requirements for mobile devices. Each licensee that possesses mobile devices containing Category 1 or Category 2 quantities of radioactive material shall:
a. Have two independent physical controls that form tangible barriers to secure the material from unauthorized removal when the device is not under direct control and constant surveillance by the licensee; and
b. For devices in or on a vehicle or trailer, unless the health and safety requirements for a site prohibit the disabling of the vehicle, the licensee shall utilize a method to disable the vehicle or trailer when not under direct control and constant surveillance by the licensee. Licensees shall not rely on the removal of an ignition key to meet this requirement.
8. Security program review.
a. Each licensee shall be responsible for the continuing effectiveness of the security program. Each licensee shall ensure that the security program is reviewed to confirm compliance with the requirements of this subsection and that comprehensive actions are taken to correct any noncompliance that is identified. The review shall include the radioactive material security program content and implementation. The review shall be conducted at least annually, not to exceed 12 months.
b. The results of the review, along with all recommendations, shall be documented. Each review report shall identify conditions that are adverse to the proper performance of the security program, the cause of the condition, corrective actions taken, and, when appropriate, recommend corrective actions. The licensee shall review the findings and take any additional corrective actions necessary to preclude repetition of the condition, including reassessment of the deficient areas where indicated.
c. The licensee shall maintain the review documentation for three years.
9. Reporting of events.
a. The licensee shall immediately notify the LLEA after determining that an unauthorized entry resulted in an actual or attempted theft, sabotage, or diversion of Category 1 or Category 2 quantity of radioactive material. As soon as possible after initiating a response, but not at the expense of causing delay or interfering with the LLEA response to the event, the licensee shall notify the agency by telephone at 804-864-8150 during normal business hours and 804-624-2400 after hours. In no case shall the notification to the agency be later than four hours after the discovery of any attempted or actual theft, sabotage, or diversion.
b. The licensee shall assess any suspicious activity related to possible theft, sabotage, or diversion of Category 1 or Category 2 quantities of radioactive material and notify the LLEA as appropriate. As soon as possible but not later than four hours after notifying the LLEA, the licensee shall notify the agency by telephone 804-864-8150 during normal business hours and 804-624-2400 after hours.
c. The initial telephonic notification shall be followed within a period of 30 days by a written report submitted to the agency. The report shall include sufficient information for agency analysis and evaluation, including identification of any necessary corrective actions to prevent future instances.
D. Physical protection in transit.
1. Additional requirements for transfer of Category 1 and Category 2 quantities of radioactive material. A licensee transferring a Category 1 or Category 2 quantity of radioactive material to a licensee of the agency, the NRC, or another agreement state shall meet the license verification provisions listed in this subdivision instead of those listed in 12VAC5-481-570.
a. Any licensee transferring Category 1 quantities of radioactive material to a licensee of the agency, the NRC, or another agreement state, prior to conducting such transfer, shall verify with the NRC's license verification system or the license issuing authority that the transferee's license authorizes the receipt of the type, form, and quantity of radioactive material to be transferred and that the licensee is authorized to receive radioactive material at the location requested for delivery. If the verification is conducted by contacting the license-issuing authority, the transferor shall document the verification. For transfers within the same organization, the licensee does not need to verify the transfer.
b. Any licensee transferring Category 2 quantities of radioactive material to a licensee of the agency, the NRC, or another agreement state, prior to conducting such transfer, shall verify with the NRC's license verification system or the license-issuing authority that the transferee's license authorizes the receipt of the type, form, and quantity of radioactive material to be transferred. If the verification is conducted by contacting the license-issuing authority, the transferor shall document the verification. For transfers within the same organization, the licensee does not need to verify the transfer.
c. In an emergency where the licensee cannot reach the license-issuing authority and the license verification system is nonfunctional, the licensee may accept a written certification by the transferee that it is authorized by license to receive the type, form, and quantity of radioactive material to be transferred. The certification shall include the license number, current revision number, issuing agency, expiration date, and for a Category 1 shipment, the authorized address. The licensee shall keep a copy of the certification. The certification shall be confirmed by use of the NRC's license verification system or by contacting the license-issuing authority by the end of the next business day.
d. The transferor shall keep a copy of the verification documentation as a record for three years.
2. Applicability of physical protection of Category 1 and Category 2 quantities of radioactive material during transit.
a. For shipments of category 1 quantities of radioactive material, each shipping licensee shall comply with the requirements for physical protection contained in subdivisions 3 a, 3 e, 4, 5 a (1), 5 b (1), 5 c, 6 a, 6 c, 6 e, 6 g, and 6 h of this subsection.
b. For shipments of Category 2 quantities of radioactive material, each shipping licensee shall comply with the requirements for physical protection contained in subdivisions 3 b through 3 e, 5 a (2), 5 a (3), 5 b (2), 5 c, 6 b, 6 d, 6 f, 6 g, and 6 h of this subsection.
c. The shipping licensee shall be responsible for meeting the requirements of this subsection unless the receiving licensee has agreed in writing to arrange for the in-transit physical protection required under this subsection.
3. Preplanning and coordination of shipment of Category 1 or Category 2 quantities of radioactive material.
a. Each licensee that plans to transport, or deliver to a carrier for transport, licensed material that is a Category 1 quantity of radioactive material outside the confines of the licensee's facility or other place of use or storage shall:
(1) Preplan and coordinate shipment arrival and departure times with the receiving licensee;
(2) Preplan and coordinate shipment information with the governor or the governor's designee of any state through which the shipment will pass to discuss the state's intention to provide law-enforcement escorts and identify safe havens; and
(3) Document the preplanning and coordination activities.
b. Each licensee that plans to transport, or deliver to a carrier for transport, licensed material that is a Category 2 quantity of radioactive material outside the confines of the licensee's facility or other place of use or storage shall coordinate the shipment no-later-than arrival time and the expected shipment arrival with the receiving licensee. The licensee shall document the coordination activities.
c. Each licensee that receives a shipment of a Category 2 quantity of radioactive material shall confirm receipt of the shipment with the originator. If the shipment has not arrived by the no-later-than arrival time, the receiving licensee shall notify the originator.
d. Each licensee that transports or plans to transport a shipment of a Category 2 quantity of radioactive material and determines that the shipment will arrive after the no-later-than arrival time provided pursuant to subdivision 3 b of this subsection, shall promptly notify the receiving licensee of the new no-later-than arrival time.
e. The licensee shall retain a copy of the documentation for preplanning and coordination and any revision thereof as a record for three years.
4. As specified in subdivision 3 of this subsection, each licensee shall provide advance notification to the agency and the governor of a state, or the governor's designee, of the shipment of licensed material in a Category 1 quantity, through or across the boundary of the state, before the transport or delivery to a carrier for transport of the licensed material outside the confines of the licensee's facility or other place of use or storage.
a. Procedures for submitting advance notification;
(1) The notification shall be made to the agency and to the office of each appropriate governor or governor's designee. The contact information, including telephone and mailing addresses, of governors and governor's designees is available on the NRC website at http://nrc-stp.ornl.gov/special/designee.pdf https://scp.nrc.gov/special/designee.pdf. The notification to the agency shall be in accordance with 12VAC5-481-150.
(2) A notification delivered by mail shall be postmarked at least seven days before transport of the shipment commences at the shipping facility.
(3) A notification delivered by any means other than mail shall reach the agency at least four days before the transport of the shipment commences and shall reach the office of the governor or the governor's designee at least four days before transport of a shipment within or through the state.
b. Each advance notification of shipment of Category 1 quantities of radioactive material shall contain the following information, if available at the time of the notification:
(1) The name, address, and telephone number of the shipper, carrier, and receiver of the Category 1 radioactive material;
(2) The license numbers of the shipper and receiver;
(3) A description of the radioactive material contained in the shipment, including the radionuclides and quantity;
(4) The point of origin of the shipment and the estimated time and date that shipment will commence;
(5) The estimated time and date that the shipment is expected to enter each state along the route;
(6) The estimated time and date of arrival for the shipment at the destination; and
(7) A point of contact, with a telephone number, for current shipment information.
c. Revision notice.
(1) The licensee shall provide any information not previously available at the time of the initial notification, as soon as the information becomes available but not later than commencement of the shipment, to the agency and the governor of the state or the governor's designee.
(2) A licensee shall promptly notify the agency and governor of the state or the governor's designee of any changes to the information provided in accordance with this subdivision.
d. Each licensee who cancels a shipment for which advance notification has been sent shall send a cancellation notice to the agency and the governor of each state or to the governor's designee previously notified. The licensee shall send the cancellation notice before the shipment would have commenced or as soon thereafter as possible. The licensee shall state in the notice that it is a cancellation and identify the advance notification that is being canceled.
e. The licensee shall retain a copy of the advance notification and any revision and cancellation notices as a record for three years.
f. State officials, state employees, and other individuals, whether or not licensees of the agency, NRC, or another agreement state, who receive schedule information of the kind specified in subdivision 4 b of this subsection shall protect that information against unauthorized disclosure as specified in subdivision C 2 d of this section.
5. Requirements for physical protection of Category 1 and Category 2 quantities of radioactive material during shipment.
a. Shipments by road.
(1) Each licensee who transports or delivers to a carrier for transport in a single shipment a Category 1 quantity of radioactive material shall:
(a) Ensure that movement control centers are established that maintain position information from a remote location. These control centers shall monitor shipments 24 hours a day, seven days a week and have the ability to communicate immediately, in an emergency, with the appropriate law-enforcement agencies;
(b) Ensure that redundant communications are established that allow the transport to contact the escort vehicle, when an escort vehicle is used, and movement control center at all times. Redundant communications may not be subject to the same interference factors as the primary communication;
(c) Ensure that shipments are continuously and actively monitored by a telemetric position monitoring system or an alternative tracking system reporting to a movement control center. A movement control center shall provide positive confirmation of the location, status, and control over the shipment. The movement control center shall be prepared to promptly implement preplanned procedures in response to deviations from the authorized route or a notification of actual, attempted, or suspicious activities related to the theft, loss, or diversion of a shipment. These procedures will include, but not be limited to, the identification of and contact information for the appropriate LLEA along the shipment route;
(d) Provide an individual to accompany the driver for those highway shipments with a driving time period greater than the maximum number of allowable hours of service in a 24-hour duty day as established by the U.S. Department of Transportation Federal Motor Carrier Safety Administration. The accompanying individual may be another driver; and
(e) Develop written normal and contingency procedures to address (i) notifications to the communication center and law-enforcement agencies; (ii) communication protocols that shall include a strategy for the use of authentication codes and duress codes and provisions for refueling and other stops, detours, and locations where communication is expected to be temporarily lost; (iii) loss of communication; and (iv) responses to an actual or attempted theft or diversion of a shipment.
(f) Each licensee who makes arrangements for the shipment of Category 1 quantities of radioactive material shall ensure that drivers, accompanying personnel, and movement control center personnel have access to the normal and contingency procedures.
(2) Each licensee that transports Category 2 quantities of radioactive material shall maintain constant control and surveillance during transit and have the capability for immediate communication to summon appropriate response or assistance.
(3) Each licensee who delivers to a carrier for transport in a single shipment a Category 2 quantity of radioactive material shall:
(a) Use carriers that have established package tracking systems. An established package tracking system is a documented, proven, and reliable system routinely used to transport objects of value. In order for a package tracking system to maintain constant control and surveillance, the package tracking system shall allow the shipper or transporter to identify when and where the package was last and when it should arrive at the next point of control;
(b) Use carriers that maintain constant control and surveillance during transit and have the capability for immediate communication to summon appropriate response or assistance; and
(c) Use carriers that have established tracking systems that require an authorized signature prior to releasing the package for delivery or return.
b. Shipments by rail.
(1) Each licensee who transports, or delivers to a carrier for transport, in a single shipment a Category 1 quantity of radioactive material shall:
(a) Ensure that rail shipments are monitored by a telemetric position monitoring system or an alternative tracking system reporting to the licensee, third-party, or railroad communications center. The communications center shall provide positive confirmation of the location of the shipment and its status. The communications center shall implement preplanned procedures in response to deviations from the authorized route or to a notification of actual, attempted, or suspicious activities related to the theft or diversion of a shipment. These procedures will include, but not be limited to, the identification of and contact information for the appropriate LLEA along the shipment route; and
(b) Ensure that periodic reports to the communications center are made at preset intervals.
(2) Each licensee who transports, or delivers to a carrier for transport, in a single shipment a Category 2 quantity of radioactive material shall:
(a) Use carriers that have established package tracking systems. An established package tracking system is a documented, proven, and reliable system routinely used to transport objects of value. In order for a package tracking system to maintain constant control and surveillance, the package tracking system shall allow the shipper or transporter to identify when and where the package was last and when it should arrive at the next point of control;
(b) Use carriers that maintain constant control and surveillance during transit and have the capability for immediate communication to summon appropriate response or assistance; and
(c) Use carriers that have established tracking systems that require an authorized signature prior to releasing the package for delivery or return.
c. Each licensee who makes arrangements for the shipment of Category 1 quantities of radioactive material shall immediately conduct an investigation upon discovery that a Category 1 shipment is lost or missing. Each licensee who makes arrangements for the shipment of Category 2 quantities of radioactive material shall immediately conduct an investigation, in coordination with the receiving licensee, of any shipment that has not arrived by the designated no-later-than arrival time.
6. Reporting of events.
a. The shipping licensee shall notify the appropriate LLEA and the agency within one hour of its determination that a shipment of Category 1 quantities of radioactive material is lost or missing. The appropriate LLEA would be the law-enforcement agency in the area of the shipment's last confirmed location. During the investigation required by this subsection, the shipping licensee will provide agreed upon updates to the agency on the status of the investigation.
b. The shipping licensee shall notify the agency within four hours of its determination that a shipment of Category 2 quantities of radioactive material is lost or missing. If, after 24 hours of its determination that the shipment is lost or missing, the radioactive material has not been located and secure, the licensee shall immediately notify the agency.
c. The shipping licensee shall notify the designated LLEA along the shipment route as soon as possible upon discovery of any actual or attempted theft of diversion of a shipment or suspicious activities related to the theft or diversion of a shipment of a Category 1 quantity of radioactive material. As soon as possible after notifying the LLEA, the licensee shall notify the agency upon discovery of any actual or attempted theft or diversion of a shipment, or any suspicious activity related to the shipment, of Category 1 radioactive material.
d. The shipping licensee shall notify the agency as soon as possible upon discovery of any actual or attempted theft or diversion of a shipment, or any suspicious activity related to the shipment, of a Category 2 quantity of radioactive material.
e. The shipping licensee shall notify the agency and the LLEA as soon as possible upon recovery of any lost or missing Category 1 quantities of radioactive material.
f. The shipping licensee shall notify the agency as soon as possible upon recovery of any lost or missing Category 2 quantities of radioactive material.
g. The initial telephonic notification required by subdivisions 6 a through 6 d of this subsection shall be followed within a period of 30 days by a written report submitted to the agency. The report shall include the following information:
(1) A description of the licensed material involved, including kind, quantity, and chemical and physical form;
(2) A description of the circumstances under which the loss or theft occurred;
(3) A statement of disposition, or probable disposition, of the licensed material involved;
(4) Actions that have been taken, or will be taken, to recover the material; and
(5) Procedures or measures that have been, or will be, adopted to ensure against a recurrence of the loss or theft of licensed material.
h. Subsequent to filing the written report, the licensee shall also report any additional substantive information on the loss or theft within 30 days after the licensee learns of such information.
E. Records.
1. Each record required by this section shall be legible throughout the retention period specified. The record may be the original or a reproduced copy or a microform, provided that the copy or microform is authenticated by authorized personnel and that the microform is capable of producing a clear copy throughout the required retention period. The record may also be stored in electronic media with the capability for producing legible, accurate, and complete records during the required retention period. Records such as letters, drawings, and specifications shall include all pertinent information such as stamps, initials, and signatures. The licensee shall maintain adequate safeguards against tampering with and loss of records.
2. Licensees shall maintain the records that are required by this section for the period specified. If a retention period is not otherwise specified, these records shall be retained until the agency terminates the facility's license. All records related to this section may be destroyed upon agency termination of the facility license.
12VAC5-481-1110. Reporting requirements.
A. Licensees shall notify the agency as soon as possible but not later than four hours after the discovery of an event that prevents immediate protective actions necessary to avoid exposures to radiation or radioactive materials that could exceed regulatory limits or releases of licensed material that could exceed regulatory limits (events may include fires, explosions, toxic gas releases, etc.). Licensees shall:
1. If required by this subsection and subsection B, notify the agency of any event, via telephone, during normal business hours to (804) 864-8150 or after hours to the State Emergency Operations Center at (804) 624-2400.
2. Submit a written report, either by mail or by hand delivery to the agency at 109 Governor Street, 7th Floor, Richmond, VA 23219.
B. Licensees shall notify the agency within 24 hours after the discovery of any of the following events involving licensed material:
1. An unplanned contamination event that:
a. Requires access to the contaminated area by workers or the public to be restricted for more than 24 hours by imposing additional radiological controls or by prohibiting entry into the area;
b. Involves a quantity of material greater than five times the lowest annual limit on intake specified in Appendix B to 10 CFR Part 20; and
c. Has access to the area restricted for a reason other than to allow isotopes with a half-life of less than 24 hours to decay prior to decontamination.
2. An event in which equipment is disabled or fails to function as designed when:
a. The equipment is required by regulation or license condition to prevent releases exceeding regulatory limits, to prevent exposures to radiation and radioactive materials exceeding regulatory limits, or to mitigate the consequences of an accident;
b. The equipment is required to be available and operable when it is disabled or fails to function; and
c. No redundant equipment is available and operable to perform the required safety function.
3. An event that requires unplanned medical treatment at a medical facility of an individual with spreadable radioactive contamination on the individual's clothing or body.
4. An unplanned fire or explosion damaging any licensed material or any device, container, or equipment containing licensed material when:
a. The quantity of material involved is greater than five times the lowest annual limit on intake specified in Appendix B to 10 CFR Part 20; and
b. The damage affects the integrity of the licensed material or its container.
C. Notifications of any event made by licensees in response to the requirements of subsections A and B of this section shall be made to the agency, via telephone, during normal business hours to (804) 864-8150 or after hours to the State Emergency Operations Center at (804) 624-2400 and provide the following:
1. To the extent that the information is available at the time of the notification, provide a name and call back telephone number;
2. A description of the event, including date and time; if known, the sequence of occurrences leading to the event including degradation or failure of structures, systems, equipment, components; and activities of personnel relied on to prevent potential accidents;
3. The exact location of the event and whether the remaining structures, systems, equipment, components, and activities of personnel relied on to mitigate the consequences are available and reliable to perform their function;
4. Radiological or chemical hazards involved including the isotopes, quantities, and chemical and physical form of the licensed material;
5. Actual or potential health and safety consequences to the workers, the public, and the environment, including relevant chemical and any radiation data for actual personnel exposures to radiation or radioactive materials or hazardous chemicals produced from licensed material;
6. External conditions affecting the event;
7. Status of the event including actions taken by the licensee in response to the event and the current and planned site status;
8. Notification, related to the event, that were made or are planned to be made to any other local, state, or federal agencies; and
9. Status of any press releases related to the event that were made or are planned.
10. Each licensee that makes a report required by subsection A or B of this section shall submit a written follow-up report within 30 days of the initial report. Written reports prepared pursuant to other regulations may be submitted to fulfill this requirement if the report contains all necessary information and the appropriate distribution is made. These written reports must be sent to the agency at 109 Governor Street, 7th Floor, Richmond, VA 23219, and must include the following:
a. All information required from the telephone notification included in this subsection;
b. The probable cause of the event, including all factors that contributed to the event and the manufacturer and model number (if applicable) of any equipment that failed or malfunctioned;
c. Corrective actions taken or planned to prevent occurrence of similar or identical events in the future and the results of any evaluations or assessments; and
d. For licenses subject to 10 CFR Part 70 Subpart H, whether the event was identified and evaluated in the integrated safety analysis.
D. In addition to the notifications required by 12VAC5-481-1100 or subsections A and B of this section, each licensee shall submit a written report within 30 days after learning of any of the following occurrences, either by mail or by hand delivery, to the agency at 109 Governor Street, 7th Floor, Richmond, VA 23219:
1. Any incident for which notification is required by 12VAC5-481-1100 or subsections A and B of this section;
2. Doses in excess of any of the following:
a. The occupational dose limits for adults in 12VAC5-481-640;
b. The occupational dose limits for a minor in 12VAC5-481-700;
c. The limits for an embryo/fetus of a declared pregnant woman in 12VAC5-481-710;
d. The limits for an individual member of the public in 12VAC5-481-720;
e. Any applicable limits in the license; or
f. The ALARA constraints for air emissions established under 12VAC5-481-630 D;
3. Levels of radiation or concentrations of radioactive material in:
a. A restricted area in excess of any applicable limit in the license; or
b. An unrestricted area in excess of 10 times any applicable limit set forth in this part or in the license, whether or not involving exposure of any individual in excess of the limits in 12VAC5-481-720; or
4. For licensee subject to the provisions of the U.S. Environmental Protection Agency's generally applicable environmental radiation standards in 40 CFR Part 190, levels of radiation or releases of radioactive materials in excess of those standards, or of license conditions related to those standards.
E. Each report, required by subsection A of this section shall:
1. Describe the extent of exposure of individuals to radiation and radioactive material, including, as appropriate:
a. A description of the event, including the probable cause, the exact location, the isotopes and quantities, chemical and physical form of the licensed material involved, date and time of the event, and if applicable, the manufacturer and model number of any equipment that failed or malfunctioned;
b. Estimates of each individual's dose;
c. The levels of radiation and concentrations of radioactive material involved;
d. The cause of the elevated exposures, dose rates, or concentrations; and
e. Corrective steps taken or planned to ensure against a recurrence, including the schedule for achieving conformance with applicable limits, ALARA constraints, generally applicable environmental standards, and associated license conditions and the results of all evaluations or assessments.
2. Include for each individual the name, social security number, and date of birth. With respect to the limit for the embryo/fetus, the identifiers should be those of the declared pregnant woman. The report shall be prepared so that this information is stated in a separate and detachable part of the report and shall be clearly labeled for protection under privacy laws.
12VAC5-481-2970. Exemptions.
A. Common and contract carriers, freight forwarders, and warehouse workers that are subject to the requirements of the United States Department of Transportation (DOT) in 49 CFR Part 170 through 49 Part CFR 189 or the United States Postal Service in the Postal Service Domestic Mail Manual (DMM), Section C-023.9.0, and the United States Postal Service, are exempt from the requirements of this part to the extent that they transport or store radioactive material in the regular course of their carriage for others or storage incident thereto. Common and contract carriers that are not subject to the requirements of the DOT or United States Postal Service are subject to 12VAC5-481-2960 and other applicable requirements of these regulations.
B. A licensee is exempt from all the requirements of this part with respect to shipment or carriage of the following low-level materials:
1. NARM Natural material and ores containing naturally occurring radionuclides that are either in their natural state, or have only been processed for purposes other than the extraction of the radionuclides, and that are not intended to be processed for use of these radionuclides, provided the activity concentration of the material does not exceed 10 times the values specified in Table A-2 2 of 12VAC5-481-3770.
2. Materials for which the activity concentration is not greater than the activity concentration values specified in Table A-2 2 or Table 3 of 12VAC5-481-3770, or for which the consignment activity is not greater than the limit for an exempt consignment found in Table A-2 2 or Table 3 of 12VAC5-481-3770.
3. Nonradioactive solid objects with radioactive substances present on any surfaces in quantities not in excess of the levels cited in the definition of "contamination" in 12VAC5-481-10.
C. Fissile material meeting one of the following requirements are exempt from classification as fissile material and from the fissile material package standards of 10 CFR 71.55 and 71.59, but are subject to all other requirements of 10 CFR 71, except as noted.
1. Individual package containing two grams or less fissile material.
2. Individual or bulk packaging containing 15 grams or less of fissile material provided the package has at least 200 grams of solid nonfissile material for every gram of fissile material. Lead, beryllium, graphite, and hydrogenous material enriched in deuterium may be present in the package but must not be included in determining the required mass for solid nonfissile material.
3. Low concentrations of solid fissile material commingled with solid nonfissile material, provided that there is at least 2,000 grams of solid nonfissile material for every gram of fissile material, and there is no more than 180 grams of fissile material distributed within 360 kg of contiguous nonfissile material. Lead, beryllium, graphite, and hydrogenous material enriched in deuterium may be present in the package but must not be included in determining the required mass of solid nonfissile material.
4. Uranium enriched in uranium-235 to a maximum of 1.0% by weight, and with total plutonium and uranium-233 content of up to 1.0% of the mass of uranium-235, provided that the mass of any beryllium, graphite, and hydrogenous material enriched in deuterium constitutes less than 5.0% of the uranium mass, and that the fissile material is distributed homogeneously and does not form a lattice arrangement within the package.
5. Liquid solutions of uranyl nitrate enriched in uranium-235 to a maximum of 2.0% by mass, with a total plutonium and uranium-233 content not exceeding 0.002% of the mass of uranium, and with a minimum nitrogen to uranium atomic ratio (N/U) of 2. The material must be contained in at least a DOT Type A package.
6. Packages containing, individually, a total plutonium mass of not more than 1,000 grams, of which not more than 20% by mass may consist of plutonium-239, plutonium-241, or any combination of these radionuclides.
D. Any physician licensed by the Commonwealth of Virginia to dispense drugs in the practice of medicine is exempt from this section with respect to transport by the physician of radioactive material for use in the practice of medicine provided the physician is an authorized user under Part VII (12VAC5-481-1660 et seq.).
12VAC5-481-3000. General license: NRC-approved packages.
A. A general license is hereby issued to any licensee to transport, or to deliver to a carrier for transport, licensed material in a package for which a license, certificate of compliance (CoC), or other approval has been issued by the NRC. This general license applies only to a licensee who has a quality assurance program approved by the agency as satisfying the provisions of 12VAC5-481-3130.
B. This general license applies only to a licensee who:
1. Has a copy of the specific license, CoC, or other approval by the NRC of the package and has the drawings and other documents referenced in the approval relating to the use and maintenance of the packaging and to the actions to be taken prior to shipment;
2. Complies with the terms and conditions of the license, certificate, or other approval by the NRC, as applicable, and the applicable requirements of Part XIII (12VAC5-481-2950 et seq.) of this chapter;
3. Prior to the licensee's first use of the package, submits in writing to the NRC agency the licensee's name and license number and the package identification number specified in the package approval using the appropriate method listed in 10 CFR 71.1(a) in accordance with 12VAC5-481-150; and
4. Has a quality assurance program that complies with 12VAC5-481-3130.
C. The general license in subsection A of this section applies only when the package approval authorizes use of the package under this general license.
D. For a Type B or fissile material package, the design of which was approved by the NRC before April 1, 1996, the general license is subject to the additional restrictions of 12VAC5-481-3010.
12VAC5-481-3030. General License: use of foreign approved package.
A. A general license is issued to any licensee to transport, or to deliver to a carrier for transport, licensed material in a package the design of which has been approved in a foreign national competent authority certificate that has been revalidated by the DOT as meeting the applicable requirements of 49 CFR 171.12 171.23.
B. This general license applies only to international shipments made to or from locations outside the United States.
C. This general license applies only to a licensee who:
1. Has a copy of the applicable certificate, the revalidation, and the drawings and other documents referenced in the certificate relating to the use and maintenance of the packaging and to the actions to be taken prior to shipment;
2. Complies with the terms and conditions of the certificate and revalidation, and with the applicable requirements of this part; and
3. The licensee has a quality assurance program approved by the agency that complies with 12VAC5-481-3130.
12VAC5-481-3070. Preliminary determinations.
Prior to the first use of any packaging for the shipment of radioactive material:, the licensee shall ascertain that the determinations made in 10 CFR 71.85(a) through (c) have been made.
1. The licensee shall ascertain that there are no cracks, pinholes, uncontrolled voids, or other defects which could significantly reduce the effectiveness of the packaging;
2. Where the maximum normal operating pressure will exceed 35 kilopascal (5 lbf/in2) gauge, the licensee shall test the containment system at an internal pressure at least 50% higher than the maximum normal operating pressure to verify the capability of that system to maintain its structural integrity at that pressure;
3. The licensee shall determine that the packaging has been fabricated in accordance with the design approved by the NRC; and
4. The licensee shall conspicuously and durably mark the packaging with its model number, serial number, gross weight, and a package identification number as assigned by the NRC.
12VAC5-481-3100. Shipment records.
A. Each licensee shall maintain for a period of three years after shipment a record of each shipment of licensed material not exempt under 12VAC5-481-2970, showing, where applicable:
1. Identification of the packaging by model number and serial number;
2. Verification that the packaging, as shipped, had no significant defect;
3. Volume and identification of coolant;
4. Type and quantity of licensed material in each package, and the total quantity of each shipment;
5. Date of the shipment;
6. Name and address of the transferee;
7. Address to which the shipment was made; and
8. Results of the determinations required by 12VAC5-481-3080 and by the conditions of the package approval.
9. For each item of irradiated fissile material, in addition to the items listed in subdivisions 1 through 8 of this subsection, identification by model number and serial number; irradiation and decay history to the extent appropriate to demonstrate that its nuclear and thermal characteristics comply with license conditions; and any abnormal or unusual condition relevant to radiation safety.
10. For each fissile package and for Type B packages, in addition to the items listed in subdivisions 1 through 8 of this subsection, any special controls exercised.
B. Each licensee shall maintain, for a period of three years after the life of the packaging to which they apply, records identifying the packaging by model number, serial number, and date of manufacture.
C. The licensee shall make available to the agency for inspection, upon reasonable notice, all records required by this section. Records are valid if stamped, initialed, or signed and dated by authorized personnel, or otherwise authenticated.
D. The licensee shall maintain sufficient written records to furnish evidence of the quality of the packaging. The records to be maintained include results of the determinations made by 12VAC5-481-3000; design, fabrication, and assembly records; results of reviews, inspections, tests, and audits; results of monitoring work performance and materials analyses; and results of maintenance, modification, and repair activities. Inspection, test, and audit records must identify the inspector or data recorder, the type of observation, the results, the acceptability, and the actions to be taken in connection with any deficiencies noted. These records must be retained for three years after the life of the packaging to which they apply.
Article 5
Quality Assurance
12VAC5-481-3130. Quality assurance.
A. Quality assurance requirements apply to the design, purchase, fabrication, handling, shipping, storing, cleaning, assembly, inspection, testing, operation, maintenance, repair, and modification of components of packaging that are important to safety. Quality assurance comprises all those planned and systematic actions necessary to provide adequate confidence that a system or component will perform satisfactorily in service. Quality assurance includes quality control, which comprises those quality assurance actions related to control of the physical characteristics and quality of the material or component to predetermined requirements. The licensee, certificate holder, and applicant for a CoC are is responsible for the quality assurance requirements as they apply to design, fabrication, testing, and modification of packaging. Each licensee is responsible for the quality assurance provision that applies to its use of packaging for the shipment of licensed material subject to this chapter.
B. Each licensee, certificate holder and applicant for a CoC shall establish, maintain, and execute a quality assurance program satisfying each of that applicable criteria of this section, 10 CFR Part 71, Subpart H and satisfying any specific provisions that are applicable to the licensee's activities including procurement of packaging. The licensee, certificate holder, and applicant for CoC shall execute the applicable criteria in a graded approach to an extent that is commensurate with the quality assurance requirement's importance to safety.
C. Before the use of any package for the shipment of licensed material subject to this rule, each licensee shall obtain NRC approval of its quality assurance program. Using an appropriate method, each licensee shall file a description of its quality assurance program, including a discussion of which requirements of this section are applicable and how they will be satisfied by submitting the description to the agency.
D. A program for transport container inspection and maintenance limited to radiographic exposure devices, source changers, or packages transporting these devices and meeting the requirements of 12VAC5-481-1270 or equivalent NRC or agreement state requirement, is deemed to satisfy the requirements of 12VAC5-481-3000 and subsection B of this section.
E. The licensee, certificate holder, and applicant for a CoC shall be responsible for the establishment and execution of the quality assurance program. The licensee, certificate holder, and applicant for a CoC may delegate to others, such as contractors, agents, or consultants, the work of establishing and executing the quality assurance program, or any part of the quality assurance program, but shall retain responsibility for the program. The licensee shall clearly establish and delineate, in writing, the authority and duties of persons and organizations performing activities affecting the safety-related functions of structures, systems, and components. These activities include performing the functions associated with attaining quality objectives and the quality assurance functions. While the term licensee is used in these criteria, the requirements are applicable to whatever design, fabrication, assembly, and testing of the package is accomplished with respect to a package before the time a package is issued.
F. The quality assurance functions are:
1. Assuring that an appropriate quality assurance program is established and effectively executed; and
2. Verifying, by procedures such as checking, auditing, and inspection, that activities affecting the safety-related functions have been performed correctly.
G. The persons and organizations performing quality assurance functions must have sufficient authority and organizational freedom to:
1. Identify quality problems;
2. Initiate, recommend, or provide solutions; and
3. Verify implementation of solutions.
H. Changes to the quality assurance program.
1. Each quality assurance program approval holder shall submit a description of a proposed change to its agreement state approved quality assurance program that will reduce commitments in the program description as approved by the agreement state. The quality assurance program approval holder shall not implement the change before receiving approval.
The description of a proposed change to the agreement state approved quality assurance program must identify the change, the reason for the change, and the basis for concluding that the revised program incorporating the change continues to satisfy the applicable requirements of this section.
2. Each quality assurance program approval holder may change a previously approved quality assurance program without prior agreement state approval if the change does not reduce the commitments in the quality assurance program previously approved by the agreement state. Changes to the quality assurance program that do not reduce the commitments shall be submitted to the agreement state every 24 months. In addition to quality assurance program changes involving administrative improvements and clarifications, spelling corrections, and nonsubstantive changes to punctuation or editorial items, the following changes are not considered reductions in commitment:
a. The use of a quality assurance standard approved by the agreement state that is more recent than the quality assurance standard in the licensee's current quality assurance program at the time of the change;
b. The use of generic organizational position titles that clearly denote the position function, supplemented as necessary by descriptive text, rather than specific titles, provided that there is no substantive change to either the functions of the position or reporting requirements;
c. The use of generic organization charts to indicate functional relationships, authorities, and responsibilities, or alternatively, the use of descriptive text, provided that there is no substantive change to the functional relationships, authorities, or responsibilities;
d. The elimination of quality assurance program information that duplicates language in quality assurance regulatory guides and quality assurance standards to which the quality assurance program approval holder has committed to on record; and
e. Organizational revisions that ensure that persons and organizations performing quality assurance functions continue to have the requisite authority and organizational freedom, including independence from cost and schedule when opposed to safety considerations.
3. Each quality assurance program approval holder shall maintain records of quality assurance program changes.
I. The licensee shall maintain sufficient written records to describe the activities affecting quality. These records must include the changes to the quality assurance program as required by subsection H of this section, the instructions, procedures, and drawings required by this section to prescribe quality assurance activities, and closely related specifications such as required qualifications of personnel, procedures, and equipment. The records must include the instructions or procedures that establish a records retention program that is consistent with applicable regulations and designates factors such as duration, location, and assigned responsibility. The licensee shall retain these records for three years beyond the date when the licensee last engaged in the activity for which the quality assurance program was developed. If any portion of the quality assurance program, written procedures or instructions is superseded, the licensee shall retain the superseded material for three years after it is superseded.
12VAC5-481-3770. Determination of A1 and A2.
A. Values of A1 and A2 for individual radionuclides, which are the bases for many activity limits elsewhere in these regulations, are given in Table 1 of this section. The curie (Ci) values specified are obtained by converting from the Terabecquerel (TBq) value. The terabecquerel values are the regulatory standard. The curie values are for information only and are not intended to be the regulatory standard. Where values of A1 and A2 are unlimited, it is for radiation control purposes only. For nuclear criticality safety, some materials are subject to controls placed on fissile material.
B. For individual radionuclides whose identities are known, but that are not listed in Table 1 or Table 2 of this section, the A1 and A2 values or exempt material activity concentration and exempt consignment activity values contained in Table 3 of this section may be used. Otherwise, the licensee shall obtain prior agency approval for radionuclides not listed in Table 1 or Table 2 of this section, before shipping the material. The licensee shall submit requests for prior approval to the agency.
C. In the calculations of A1 and A2 for a radionuclide not in Table 1 of this section, a single radioactive decay chain, in which radionuclides are present in their naturally occurring proportions, and in which no daughter radionuclide has a half-life either longer than 10 days, or longer than that of the parent radionuclide, shall be considered as a single radionuclide, and the activity to be taken into account, and the A1 or A2 value to be applied, shall be those corresponding to the parent radionuclide of that chain. In the case of radioactive decay chains in which any daughter radionuclide has a half-life either longer than 10 days or greater than that of the parent radionuclide, the parent and those daughter radionuclides shall be considered as mixtures of different radionuclides.
D. For mixtures of radionuclides whose identities and respective activities are known, the following conditions apply:
1. For special form radioactive material, the maximum quantity transported in a Type A package is as follows:
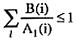
where B(i) is the activity of radionuclide (i) in special form, and A1(i) is the A1 value for radionuclide (i).
2. For normal form radioactive material, the maximum quantity transported in a Type A package is as follows:
∑B(i)/A2(i)≤1 ∑B(i)/A2(i)≤1
where B(i) is the activity of radionuclide (i) in normal form, and A2(i) is the A2 value for radionuclide (i) in special form.
3. If the package contains both special and normal form radioactive material, the activity that may be transported in the Type A package is as follows:
∑B(i)\A1(i)+∑C(j)\A2(j)≤1
Where B(i) is the activity of radionuclide i (i) as special form radioactive material, A1(i) is the A1 value for the radionuclide (i), C(j) is the activity of radionuclide (j) as normal form radioactive material, and A2(j) is the A2 value for radionuclide (j).
4. Alternatively, the A1 value for mixtures of special form material may be determined as follows:
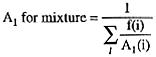
where f(i) is the fraction of activity for radionuclide (i) in the mixture, and A1(i) is the appropriate A1 value for radionuclide (i).
4. 5. Alternatively, the A2 value for mixtures of normal form material may be determined as follows:
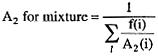
where f(i) is the fraction of activity for radionuclide (i) in the mixture, and A2(i) is the appropriate A2 value for radionuclide (i).
5. 6. The exempt activity concentration for mixtures of nuclides may be determined as follows:

where f(i) is the fraction of activity concentration of radionuclide (i) in the mixture, and [A](i) is the activity concentration for exempt material containing radionuclide (i).
6. 7. The activity limit for an exempt consignment for mixtures of radionuclides may be determined as follows:
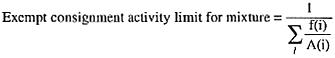
where f(i) is the fraction of activity of radionuclide (i) in the mixture, and [A](i) is the activity limit for exempt consignments for radionuclide (i).
E. When the identity of each radionuclide is known, but the individual activities of some of the radionuclides are not known, the radionuclides may be grouped, and the lowest A1 or A2 value or lowest [A] (activity concentration for exempt material or A (activity limit for exempt consignment) value, as appropriate, for the radionuclides in each group may be used in applying the formulas in subsection D of this section. Groups may be based on the total alpha activity and the total beta/gamma activity when these are known, using the lowest A1 or A2 values or the lowest [A] or A value, as appropriate, for the alpha emitters and beta/gamma emitters.
F. Table 1. A1 and A2 Values for Radionuclides.
Symbol of radionuclide | Element and atomic number | A1 (TBq) | A1(Ci)b | A2 (TBq) | A2(Ci)b | Specific activity |
(TBq/g) | (Ci/g) |
Ac-225 (a) | Actinium (89) | 8.0X10-1 | 2.2X10-1 | 6.0X10-3 | 1.6X10-1 | 2.1X103 | 5.8X104 |
Ac-227 (a) | | 9.0X10-1 | 2.4X10-1 | 9.0X10-5 | 2.4X10-3 | 2.7 | 7.2X101 |
Ac-228 | | 6.0X10-1 | 1.6X101 | 5.0X10-1 | 1.4X101 | 8.4X104 | 2.2X106 |
Ag-105 | Silver (47) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 1.1X103 | 3.0X104 |
Ag-108m (a) | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 9.7X10-1 | 2.6X101 |
Ag-110m (a) | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 1.8X102 | 4.7X103 |
Ag-111 | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 5.8X103 | 1.6X105 |
Al-26 | Aluminum (13) | 1.0X10-1 | 2.7 | 1.0X10-1 | 2.7 | 7.0X10-4 | 1.9X10-2 |
Am-241 | Americium (95) | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 1.3X10-1 | 3.4 |
Am-242m (a) | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 3.6X10-1 | 1.0X101 |
Am-243 (a) | | 5.0 | 1.4X102 | 1.0X10-3 | 2.7X10-2 | 7.4X10-3 | 2.0X10-1 |
Ar-37 | Argon (18) | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 3.7X103 | 9.9X104 |
Ar-39 | | 4.0X101 | 1.1X103 | 2.0X101 | 5.4X102 | 1.3 | 3.4X101 |
Ar-41 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.5X106 | 4.2X107 |
As-72 | Arsenic (33) | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 6.2X104 | 1.7X106 |
As-73 | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 8.2X102 | 2.2X104 |
As-74 | | 1.0 | 2.7X101 | 9.0X10-1 | 2.4X101 | 3.7X103 | 9.9X104 |
As-76 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 5.8X104 | 1.6X106 |
As-77 | | 2.0X101 | 5.4X102 | 7.0X10-1 | 1.9X101 | 3.9X104 | 1.0X106 |
At-211 (a) | Astatine (85) | 2.0X101 | 5.4X102 | 5.0X10-1 | 1.4X101 | 7.6X104 | 2.1X106 |
Au-193 | Gold (79) | 7.0 | 1.9X102 | 2.0 | 5.4X101 | 3.4X104 | 9.2X105 |
Au-194 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 1.5X104 | 4.1X105 |
Au-195 | | 1.0X101 | 2.7X102 | 6.0 | 1.6X102 | 1.4X102 | 3.7X103 |
Au-198 | | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 9.0X103 | 2.4X105 |
Au-199 | | 1.0X101 | 2.7X102 | 6.0X10-1 | 1.6X101 | 7.7X103 | 2.1X105 |
Ba-131 (a) | Barium (56) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 3.1X103 | 8.4X104 |
Ba-133 | | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 9.4 | 2.6X102 |
Ba-133m | | 2.0X101 | 5.4X102 | 6.0X10-1 | 1.6X101 | 2.2X104 | 6.1X105 |
Ba-140 (a) | | 5.0X10-1 | 1.4X101 | 3.0X10-1 | 8.1 | 2.7X103 | 7.3X104 |
Be-7 | Beryllium (4) | 2.0X101 | 5.4X102 | 2.0X101 | 5.4X102 | 1.3X104 | 3.5X105 |
Be-10 | | 4.0X101 | 1.1X103 | 6.0X10-1 | 1.6X101 | 8.3X10-4 | 2.2X10-2 |
Bi-205 | Bismuth (83) | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 1.5X103 | 4.2X104 |
Bi-206 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 3.8X103 | 1.0X105 |
Bi-207 | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 1.9 | 5.2X101 |
Bi-210 | | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 4.6X103 | 1.2X105 |
Bi-210m (a) | | 6.0X10-1 | 1.6X101 | 2.0X10-2 | 5.4X10-1 | 2.1X10-5 | 5.7X10-4 |
Bi-212 (a) | | 7.0X10-1 | 1.9X101 | 6.0X10-1 | 1.6X101 | 5.4X105 | 1.5X107 |
Bk-247 | Berkelium (97) | 8.0 | 2.2X102 | 8.0X10-4 | 2.2X10-2 | 3.8X10-2 | 1.0 |
Bk-249 (a) | | 4.0X101 | 1.1X103 | 3.0X10-1 | 8.1 | 6.1X101 | 1.6X103 |
Br-76 | Bromine (35) | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 9.4X104 | 2.5X106 |
Br-77 | | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 2.6X104 | 7.1X105 |
Br-82 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 4.0X104 | 1.1X106 |
C-11 | Carbon (6) | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 3.1X107 | 8.4X108 |
C-14 | | 4.0X101 | 1.1X103 | 3.0 | 8.1X101 | 1.6X10-1 | 4.5 |
Ca-41 | Calcium (20) | Unlimited | Unlimited | Unlimited | Unlimited | 3.1X10-3 | 8.5X10-2 |
Ca-45 | | 4.0X101 | 1.1X103 | 1.0 | 2.7X101 | 6.6X102 | 1.8X104 |
Ca-47 (a) | | 3.0 | 8.1X101 | 3.0X10-1 | 8.1 | 2.3X104 | 6.1X105 |
Cd-109 | Cadmium (48) | 3.0X101 | 8.1X102 | 2.0 | 5.4X101 | 9.6X101 | 2.6X103 |
Cd-113m | | 4.0X101 | 1.1X103 | 5.0X10-1 | 1.4X101 | 8.3 | 2.2X102 |
Cd-115 (a) | | 3.0 | 8.1X101 | 4.0X10-1 | 1.1X101 | 1.9X104 | 5.1X105 |
Cd-115m | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 9.4X102 | 2.5X104 |
Ce-139 | Cerium (58) | 7.0 | 1.9X102 | 2.0 | 5.4X101 | 2.5X102 | 6.8X103 |
Ce-141 | | 2.0X101 | 5.4X102 | 6.0X10-1 | 1.6X101 | 1.1X103 | 2.8X104 |
Ce-143 | | 9.0X10-1 | 2.4X101 | 6.0X10-1 | 1.6X101 | 2.5X104 | 6.6X105 |
Ce-144 (a) | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 1.2X102 | 3.2X103 |
Cf-248 | Californium (98) | 4.0X101 | 1.1X103 | 6.0X10-3 | 1.6X10-1 | 5.8X101 | 1.6X103 |
Cf-249 | | 3.0 | 8.1X101 | 8.0X10-4 | 2.2X10-2 | 1.5X10-1 | 4.1 |
Cf-250 | | 2.0X101 | 5.4X102 | 2.0X10-3 | 5.4X10-2 | 4.0 | 1.1X102 |
Cf-251 | | 7.0 | 1.9X102 | 7.0X10-4 | 1.9X10-2 | 5.9X10-2 | 1.6 |
Cf-252 (h) | | 5.0X10-2 1.0X10-1
| 1.4 2.7
| 3.0X10-3 | 8.1X10-2 | 2.0X101 | 5.4X102 |
Cf-253 (a) | | 4.0X101 | 1.1X103 | 4.0X10-2 | 1.1 | 1.1X103 | 2.9X104 |
Cf-254 | | 1.0X10-3 | 2.7X10-2 | 1.0X10-3 | 2.7X10-2 | 3.1X102 | 8.5X103 |
Cl-36 | Chlorine (17) | 1.0X101 | 2.7X102 | 6.0X10-1 | 1.6X101 | 1.2X10-3 | 3.3X10-2 |
Cl-38 | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 4.9X106 | 1.3X108 |
Cm-240 | Curium (96) | 4.0X101 | 1.1X103 | 2.0X10-2 | 5.4X10-1 | 7.5X102 | 2.0X104 |
Cm-241 | | 2.0 | 5.4X101 | 1.0 | 2.7X101 | 6.1X102 | 1.7X104 |
Cm-242 | | 4.0X101 | 1.1X103 | 1.0X10-2 | 2.7X10-1 | 1.2X102 | 3.3X103 |
Cm-243 | | 9.0 | 2.4X102 | 1.0X10-3 | 2.7X10-2 | 1.9X10-3 | 5.2X104 |
Cm-244 | | 2.0X101 | 5.4X102 | 2.0X10-3 | 5.4X10-2 | 3.0 | 8.1X101 |
Cm-245 | | 9.0 | 2.4X102 | 9.0X10-4 | 2.4X10-2 | 6.4X10-3 | 1.7X10-1 |
Cm-246 | | 9.0 | 2.4X102 | 9.0X10-4 | 2.4X10-2 | 1.1X10-2 | 3.1X10-1 |
Cm-247 (a) | | 3.0 | 8.1X101 | 1.0X10-3 | 2.7X10-2 | 3.4X10-6 | 9.3X10-5 |
Cm-248 | | 2.0X10-2 | 5.4X10-1 | 3.0X10-4 | 8.1X10-3 | 1.6X10-4 | 4.2X10-3 |
Co-55 | Cobalt (27) | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 1.1X105 | 3.1X106 |
Co-56 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.1X103 | 3.0X104 |
Co-57 | | 1.0X101 | 2.7X102 | 1.0X101 | 2.7X102 | 3.1X102 | 8.4X103 |
Co-58 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 1.2X103 | 3.2X104 |
Co-58m | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 2.2X105 | 5.9X106 |
Co-60 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 4.2X101 | 1.1X103 |
Cr-51 | Chromium (24) | 3.0X101 | 8.1X102 | 3.0X101 | 8.1X102 | 3.4X103 | 9.2X104 |
Cs-129 | Cesium (55) | 4.0 | 1.1X102 | 4.0 | 1.1X102 | 2.8X104 | 7.6X105 |
Cs-131 | | 3.0X101 | 8.1X102 | 3.0X101 | 8.1X102 | 3.8X103 | 1.0X105 |
Cs-132 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 5.7X103 | 1.5X105 |
Cs-134 | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 4.8X101 | 1.3X103 |
Cs-134m | | 4.0X101 | 1.1X103 | 6.0X10-1 | 1.6X101 | 3.0X105 | 8.0X106 |
Cs-135 | | 4.0X101 | 1.1X103 | 1.0 | 2.7X101 | 4.3X10-5 | 1.2X10-3 |
Cs-136 | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 2.7X103 | 7.3X104 |
Cs-137 (a) | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 3.2 | 8.7X101 |
Cu-64 | Copper (29) | 6.0 | 1.6X102 | 1.0 | 2.7X101 | 1.4X105 | 3.9X106 |
Cu-67 | | 1.0X101 | 2.7X102 | 7.0X10-1 | 1.9X101 | 2.8X104 | 7.6X105 |
Dy-159 | Dysprosium (66) | 2.0X101 | 5.4X102 | 2.0X101 | 5.4X102 | 2.1X102 | 5.7X103 |
Dy-165 | | 9.0X10-1 | 2.4X101 | 6.0X10-1 | 1.6X101 | 3.0X105 | 8.2X106 |
Dy-166 (a) | | 9.0X10-1 | 2.4X101 | 3.0X10-1 | 8.1 | 8.6X103 | 2.3X105 |
Er-169 | Erbium (68) | 4.0X101 | 1.1X103 | 1.0 | 2.7X101 | 3.1X103 | 8.3X104 |
Er-171 | | 8.0X10-1 | 2.2X101 | 5.0X10-1 | 1.4X101 | 9.0X104 | 2.4X106 |
Eu-147 | Europium (63) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 1.4X103 | 3.7X104 |
Eu-148 | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 6.0X102 | 1.6X104 |
Eu-149 | | 2.0X101 | 5.4X102 | 2.0X101 | 5.4X102 | 3.5X102 | 9.4X103 |
Eu-150 (short lived) | | 2.0 | 5.4X101 | 7.0X10-1 | 1.9X101 | 6.1X104 | 1.6X106 |
Eu-150 (long lived) | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 6.1X104 | 1.6X106 |
Eu-152 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 6.5 | 1.8X102 |
Eu-152m | | 8.0X10-1 | 2.2X101 | 8.0X10-1 | 2.2X101 | 8.2X104 | 2.2X106 |
Eu-154 | | 9.0X10-1 | 2.4X101 | 6.0X10-1 | 1.6X101 | 9.8 | 2.6X102 |
Eu-155 | | 2.0X101 | 5.4X102 | 3.0 | 8.1X101 | 1.8X101 | 4.9X102 |
Eu-156 | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 2.0X103 | 5.5X104 |
F-18 | Fluorine (9) | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 3.5X106 | 9.5X107 |
Fe-52 (a) | Iron (26) | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 2.7X105 | 7.3X106 |
Fe-55 | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 8.8X101 | 2.4X103 |
Fe-59 | | 9.0X10-1 | 2.4X101 | 9.0X10-1 | 2.4X101 | 1.8X103 | 5.0X104 |
Fe-60 (a) | | 4.0X101 | 1.1X103 | 2.0X10-1 | 5.4 | 7.4X10-4 | 2.0X10-2 |
Ga-67 | Gallium (31) | 7.0 | 1.9X102 | 3.0 | 8.1X101 | 2.2X104 | 6.0X105 |
Ga-68 | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 1.5X106 | 4.1X107 |
Ga-72 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 1.1X105 | 3.1X106 |
Gd-146 (a) | Gadolinium (64) | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 6.9X102 | 1.9X104 |
Gd-148 | | 2.0X101 | 5.4X102 | 2.0X10-3 | 5.4X10-2 | 1.2 | 3.2X101 |
Gd-153 | | 1.0X101 | 2.7X102 | 9.0 | 2.4X102 | 1.3X102 | 3.5X103 |
Gd-159 | | 3.0 | 8.1X101 | 6.0X10-1 | 1.6X101 | 3.9X104 | 1.1X106 |
Ge-68 (a) | Germanium (32) | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 2.6X102 | 7.1X103 |
Ge-71 | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 5.8X103 | 1.6X105 |
Ge-77 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.3X105 | 3.6X106 |
Hf-172 (a) | Hafnium (72) | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 4.1X101 | 1.1X103 |
Hf-175 | | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 3.9X102 | 1.1X104 |
Hf-181 | | 2.0 | 5.4X101 | 5.0X10-1 | 1.4X101 | 6.3X102 | 1.7X104 |
Hf-182 | | Unlimited | Unlimited | Unlimited | Unlimited | 8.1X10-6 | 2.2X10-4 |
Hg-194 (a) | Mercury (80) | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 1.3X10-1 | 3.5 |
Hg-195m (a) | | 3.0 | 8.1X101 | 7.0X10-1 | 1.9X101 | 1.5X104 | 4.0X105 |
Hg-197 | | 2.0X101 | 5.4X102 | 1.0X101 | 2.7X102 | 9.2X103 | 2.5X105 |
Hg-197m | | 1.0X101 | 2.7X102 | 4.0X10-1 | 1.1X101 | 2.5X104 | 6.7X105 |
Hg-203 | | 5.0 | 1.4X102 | 1.0 | 2.7X101 | 5.1X102 | 1.4X104 |
Ho-166 | Holmium (67) | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 2.6X104 | 7.0X105 |
Ho-166m | | 6.0X10-1 | 1.6X101 | 5.0X10-1 | 1.4X101 | 6.6X10-2 | 1.8 |
I-123 | Iodine (53) | 6.0 | 1.6X102 | 3.0 | 8.1X101 | 7.1X104 | 1.9X106 |
I-124 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 9.3X103 | 2.5X105 |
I-125 | | 2.0X101 | 5.4X102 | 3.0 | 8.1X101 | 6.4X102 | 1.7X104 |
I-126 | | 2.0 | 5.4X101 | 1.0 | 2.7X101 | 2.9X103 | 8.0X104 |
I-129 | | Unlimited | Unlimited | Unlimited | Unlimited | 6.5X10-6 | 1.8X10-4 |
I-131 | | 3.0 | 8.1X101 | 7.0X10-1 | 1.9X101 | 4.6X103 | 1.2X105 |
I-132 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 3.8X105 | 1.0X107 |
I-133 | | 7.0X10-1 | 1.9X101 | 6.0X10-1 | 1.6X101 | 4.2X104 | 1.1X106 |
I-134 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 9.9X105 | 2.7X107 |
I-135 (a) | | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 1.3X105 | 3.5X106 |
In-111 | Indium (49) | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 1.5X104 | 4.2X105 |
In-113m | | 4.0 | 1.1X102 | 2.0 | 5.4X101 | 6.2X105 | 1.7X107 |
In-114m (a) | | 1.0X101 | 2.7X102 | 5.0X10-1 | 1.4X101 | 8.6X102 | 2.3X104 |
In-115m | | 7.0 | 1.9X102 | 1.0 | 2.7X101 | 2.2X105 | 6.1X106 |
Ir-189 (a) | Iridium (77) | 1.0X101 | 2.7X102 | 1.0X101 | 2.7X102 | 1.9X103 | 5.2X104 |
Ir-190 | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 2.3X103 | 6.2X104 |
Ir-192 (c) | | 1.0 (c) | 2.7X101 (c) | 6.0X10-1 | 1.6X101 | 3.4X102 | 9.2X103 |
Ir-194 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 3.1X104 | 8.4X105 |
K-40 | Potassium (19) | 9.0X10-1 | 2.4X101 | 9.0X10-1 | 2.4X101 | 2.4X10-7 | 6.4X10-6 |
K-42 | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 2.2X105 | 6.0X106 |
K-43 | | 7.0X10-1 | 1.9X101 | 6.0X10-1 | 1.6X101 | 1.2X105 | 3.3X106 |
Kr-79 | Krypton (36) | 4.0 | 1.1X102 | 2.0 | 5.4X101 | 4.2X104 | 1.1X106 |
Kr-81 | Krypton (36)
| 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 7.8X10-4 | 2.1X10-2 |
Kr-85 | | 1.0X101 | 2.7X102 | 1.0X101 | 2.7X102 | 1.5X101 | 3.9X102 |
Kr-85m | | 8.0 | 2.2X102 | 3.0 | 8.1X101 | 3.0X105 | 8.2X106 |
Kr-87 | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 1.0X106 | 2.8X107 |
La-137 | Lanthanum (57) | 3.0X101 | 8.1X102 | 6.0 | 1.6X102 | 1.6X10-3 | 4.4X10-2 |
La-140 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 2.1X104 | 5.6X105 |
Lu-172 | Lutetium (71) | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 4.2X103 | 1.1X105 |
Lu-173 | | 8.0 | 2.2X102 | 8.0 | 2.2X102 | 5.6X101 | 1.5X103 |
Lu-174 | | 9.0 | 2.4X102 | 9.0 | 2.4X102 | 2.3X101 | 6.2X102 |
Lu-174m | | 2.0X101 | 5.4X102 | 1.0X101 | 2.7X102 | 2.0X102 | 5.3X103 |
Lu-177 | | 3.0X101 | 8.1X102 | 7.0X10-1 | 1.9X101 | 4.1X103 | 1.1X105 |
Mg-28 (a) | Magnesium (12) | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 2.0X105 | 5.4X106 |
Mn-52 | Manganese (25) | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.6X104 | 4.4X105 |
Mn-53 | | Unlimited | Unlimited | Unlimited | Unlimited | 6.8X10-5 | 1.8X10-3 |
Mn-54 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 2.9X102 | 7.7X103 |
Mn-56 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 8.0X105 | 2.2X107 |
Mo-93 | Molybdenum (42) | 4.0X101 | 1.1X103 | 2.0X101 | 5.4X102 | 4.1X10-2 | 1.1 |
Mo-99 (a) (i) (h) | | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 1.8X104 | 4.8X105 |
N-13 | Nitrogen (7) | 9.0X10-1 | 2.4X101 | 6.0X10-1 | 1.6X101 | 5.4X107 | 1.5X109 |
Na-22 | Sodium (11) | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 2.3X102 | 6.3X103 |
Na-24 | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 3.2X105 | 8.7X106 |
Nb-93m | Niobium (41) | 4.0X101 | 1.1X103 | 3.0X101 | 8.1X102 | 8.8 | 2.4X102 |
Nb-94 | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 6.9X10-3 | 1.9X10-1 |
Nb-95 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 1.5X103 | 3.9X104 |
Nb-97 | | 9.0X10-1 | 2.4X101 | 6.0X10-1 | 1.6X101 | 9.9X105 | 2.7X107 |
Nd-147 | Neodymium (60) | 6.0 | 1.6X102 | 6.0X10-1 | 1.6X101 | 3.0X103 | 8.1X104 |
Nd-149 | | 6.0X10-1 | 1.6X101 | 5.0X10-1 | 1.4X101 | 4.5X105 | 1.2X107 |
Ni-59 | Nickel (28) | Unlimited | Unlimited | Unlimited | Unlimited | 3.0X10-3 | 8.0X10-2 |
Ni-63 | | 4.0X101 | 1.1X103 | 3.0X101 | 8.1X102 | 2.1 | 5.7X101 |
Ni-65 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 7.1X105 | 1.9X107 |
Np-235 | Neptunium (93) | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 5.2X101 | 1.4X103 |
Np-236 (short-lived) | | 2.0X101 | 5.4X102 | 2.0 | 5.4X101 | 4.7X10-4 | 1.3X10-2 |
Np-236 (long-lived) | | 9.0X100 | 2.4X102 | 2.0X10-2 | 5.4X10-1 | 4.7X10-4 | 1.3X10-2 |
Np-237 | | 2.0X101 | 5.4X102 | 2.0X10-3 | 5.4X10-2 | 2.6X10-5 | 7.1X10-4 |
Np-239 | | 7.0 | 1.9X102 | 4.0X10-1 | 1.1X101 | 8.6X103 | 2.3X105 |
Os-185 | Osmium (76) | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 2.8X102 | 7.5X103 |
Os-191 | | 1.0X101 | 2.7X102 | 2.0 | 5.4X101 | 1.6X103 | 4.4X104 |
Os-191m | | 4.0X101 | 1.1X103 | 3.0X101 | 8.1X102 | 4.6X104 | 1.3X106 |
Os-193 | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 2.0X104 | 5.3X105 |
Os-194 (a) | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.1X101 | 3.1X102 |
P-32 | Phosphorus (15) | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 1.1X104 | 2.9X105 |
P-33 | | 4.0X101 | 1.1X103 | 1.0 | 2.7X101 | 5.8X103 | 1.6X105 |
Pa-230 (a) | Protactinium (91) | 2.0 | 5.4X101 | 7.0X10-2 | 1.9 | 1.2X103 | 3.3X104 |
Pa-231 | | 4.0 | 1.1X102 | 4.0X10-4 | 1.1X10-2 | 1.7X10-3 | 4.7X10-2 |
Pa-233 | | 5.0 | 1.4X102 | 7.0X10-1 | 1.9X101 | 7.7X102 | 2.1X104 |
Pb-201 | Lead (82) | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 6.2X104 | 1.7X106 |
Pb-202 | | 4.0X101 | 1.1X103 | 2.0X101 | 5.4X102 | 1.2X10-4 | 3.4X10-3 |
Pb-203 | | 4.0 | 1.1X102 | 3.0 | 8.1X101 | 1.1X104 | 3.0X105 |
Pb-205 | | Unlimited | Unlimited | Unlimited | Unlimited | 4.5X10-6 | 1.2X10-4 |
Pb-210 (a) | | 1.0 | 2.7X101 | 5.0X10-2 | 1.4 | 2.8 | 7.6X101 |
Pb-212 (a) | | 7.0X10-1 | 1.9X101 | 2.0X10-1 | 5.4 | 5.1X104 | 1.4X106 |
Pd-103 (a) | Palladium (46) | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 2.8X103 | 7.5X104 |
Pd-107 | | Unlimited | Unlimited | Unlimited | Unlimited | 1.9X10-5 | 5.1X10-4 |
Pd-109 | | 2.0 | 5.4X101 | 5.0X10-1 | 1.4X101 | 7.9X104 | 2.1X106 |
Pm-143 | Promethium (61) | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 1.3X102 | 3.4X103 |
Pm-144 | | 7.0X10-1 | 1.9X101 | 7.0X10-1 | 1.9X101 | 9.2X101 | 2.5X103 |
Pm-145 | | 3.0X101 | 8.1X102 | 1.0X101 | 2.7X102 | 5.2 | 1.4X102 |
Pm-147 | | 4.0X101 | 1.1X103 | 2.0 | 5.4X101 | 3.4X101 | 9.3X102 |
Pm-148m (a) | | 8.0X10-1 | 2.2X101 | 7.0X10-1 | 1.9X101 | 7.9X102 | 2.1X104 |
Pm-149 | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 1.5X104 | 4.0X105 |
Pm-151 | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 2.7X104 | 7.3X105 |
Po-210 | Polonium (84) | 4.0X101 | 1.1X103 | 2.0X10-2 | 5.4X10-1 | 1.7X102 | 4.5X103 |
Pr-142 | Praseodymium (59) | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 4.3X104 | 1.2X106 |
Pr-143 | | 3.0 | 8.1X101 | 6.0X10-1 | 1.6X101 | 2.5X103 | 6.7X104 |
Pt-188 (a) | Platinum (78) | 1.0 | 2.7X101 | 8.0X10-1 | 2.2X101 | 2.5X103 | 6.8X104 |
Pt-191 | | 4.0 | 1.1X102 | 3.0 | 8.1X101 | 8.7X103 | 2.4X105 |
Pt-193 | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 1.4 | 3.7X101 |
Pt-193m | | 4.0X101 | 1.1X103 | 5.0X10-1 | 1.4X101 | 5.8X103 | 1.6X105 |
Pt-195m | | 1.0X101 | 2.7X102 | 5.0X10-1 | 1.4X101 | 6.2X103 | 1.7X105 |
Pt-197 | | 2.0X101 | 5.4X102 | 6.0X10-1 | 1.6X101 | 3.2X104 | 8.7X105 |
Pt-197m | | 1.0X101 | 2.7X102 | 6.0X10-1 | 1.6X101 | 3.7X105 | 1.0X107 |
Pu-236 | Plutonium (94) | 3.0X101 | 8.1X102 | 3.0X10-3 | 8.1X10-2 | 2.0X101 | 5.3X102 |
Pu-237 | | 2.0X101 | 5.4X102 | 2.0X101 | 5.4X102 | 4.5X102 | 1.2X104 |
Pu-238 | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 6.3X10-1 | 1.7X101 |
Pu-239 | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 2.3X10-3 | 6.2X10-2 |
Pu-240 | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 8.4X10-3 | 2.3X10-1 |
Pu-241 (a) | | 4.0X101 | 1.1X103 | 6.0X10-2 | 1.6 | 3.8 | 1.0X102 |
Pu-242 | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 1.5X10-4 | 3.9X10-3 |
Pu-244 (a) | | 4.0X10-1 | 1.1X101 | 1.0X10-3 | 2.7X10-2 | 6.7X10-7 | 1.8X10-5 |
Ra-223 (a) | Radium (88) | 4.0X10-1 | 1.1X101 | 7.0X10-3 | 1.9X10-1 | 1.9X103 | 5.1X104 |
Ra-224 (a) | | 4.0X10-1 | 1.1X101 | 2.0X10-2 | 5.4X10-1 | 5.9X103 | 1.6X105 |
Ra-225 (a) | | 2.0X10-1 | 5.4 | 4.0X10-3 | 1.1X10-1 | 1.5X103 | 3.9X104 |
Ra-226 (a) | | 2.0X10-1 | 5.4 | 3.0X10-3 | 8.1X10-2 | 3.7X10-2 | 1.0 |
Ra-228 (a) | | 6.0X10-1 | 1.6X101 | 2.0X10-2 | 5.4X10-1 | 1.0X101 | 2.7X102 |
Rb-81 | Rubidium (37) | 2.0 | 5.4X101 | 8.0X10-1 | 2.2X101 | 3.1X105 | 8.4X106 |
Rb-83 (a) | | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 6.8X102 | 1.8X104 |
Rb-84 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 1.8X103 | 4.7X104 |
Rb-86 | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 3.0X103 | 8.1X104 |
Rb-87 | | Unlimited | Unlimited | Unlimited | Unlimited | 3.2X10-9 | 8.6X10-8 |
Rb(nat) | | Unlimited | Unlimited | Unlimited | Unlimited | 6.7X106 | 1.8X108 |
Re-184 | Rhenium (75) | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 6.9X102 | 1.9X104 |
Re-184m | | 3.0 | 8.1X101 | 1.0 | 2.7X101 | 1.6X102 | 4.3X103 |
Re-186 | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 6.9X103 | 1.9X105 |
Re-187 | | Unlimited | Unlimited | Unlimited | Unlimited | 1.4X10-9 | 3.8X10-8 |
Re-188 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 3.6X104 | 9.8X105 |
Re-189 (a) | | 3.0 | 8.1X101 | 6.0X10-1 | 1.6X101 | 2.5X104 | 6.8X105 |
Re(nat) | | Unlimited | Unlimited | Unlimited | Unlimited | 0.0 | 2.4X10-8 |
Rh-99 | Rhodium (45) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 3.0X103 | 8.2X104 |
Rh-101 | | 4.0 | 1.1X102 | 3.0 | 8.1X101 | 4.1X101 | 1.1X103 |
Rh-102 | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 4.5X101 | 1.2X103 |
Rh-102m | | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 2.3X102 | 6.2X103 |
Rh-103m | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 1.2X106 | 3.3X107 |
Rh-105 | | 1.0X101 | 2.7X102 | 8.0X10-1 | 2.2X101 | 3.1X104 | 8.4X105 |
Rn-222 (a) | Radon (86) | 3.0X10-1 | 8.1 | 4.0X10-3 | 1.1X10-1 | 5.7X103 | 1.5X105 |
Ru-97 | Ruthenium (44) | 5.0 | 1.4X102 | 5.0 | 1.4X102 | 1.7X104 | 4.6X105 |
Ru-103 (a) | | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 1.2X103 | 3.2X104 |
Ru-105 | | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 2.5X105 | 6.7X106 |
Ru-106 (a) | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 1.2X102 | 3.3X103 |
S-35 | Sulphur (16) | 4.0X101 | 1.1X103 | 3.0 | 8.1X101 | 1.6X103 | 4.3X104 |
Sb-122 | Antimony (51) | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 1.5X104 | 4.0X105 |
Sb-124 | | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 6.5X102 | 1.7X104 |
Sb-125 | | 2.0 | 5.4X101 | 1.0 | 2.7X101 | 3.9X101 | 1.0X103 |
Sb-126 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 3.1X103 | 8.4X104 |
Sc-44 | Scandium (21) | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 6.7X105 | 1.8X107 |
Sc-46 | | 5.0X10-1 | 1.4X101 | 5.0X10-1 | 1.4X101 | 1.3X103 | 3.4X104 |
Sc-47 | | 1.0X101 | 2.7X102 | 7.0X10-1 | 1.9X101 | 3.1X104 | 8.3X105 |
Sc-48 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 5.5X104 | 1.5X106 |
Se-75 | Selenium (34) | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 5.4X102 | 1.5X104 |
Se-79 | | 4.0X101 | 1.1X103 | 2.0 | 5.4X101 | 2.6X10-3 | 7.0X10-2 |
Si-31 | Silicon (14) | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 1.4X106 | 3.9X107 |
Si-32 | | 4.0X101 | 1.1X103 | 5.0X10-1 | 1.4X101 | 3.9 | 1.1X102 |
Sm-145 | Samarium (62) | 1.0X101 | 2.7X102 | 1.0X101 | 2.7X102 | 9.8X101 | 2.6X103 |
Sm-147 | | Unlimited | Unlimited | Unlimited | Unlimited | 8.5X10-1 | 2.3X10-8 |
Sm-151 | | 4.0X101 | 1.1X103 | 1.0X101 | 2.7X102 | 9.7X10-1 | 2.6X101 |
Sm-153 | | 9.0 | 2.4X102 | 6.0X10-1 | 1.6X101 | 1.6X104 | 4.4X105 |
Sn-113 (a) | Tin (50) | 4.0 | 1.1X102 | 2.0 | 5.4X101 | 3.7X102 | 1.0X104 |
Sn-117m | | 7.0 | 1.9X102 | 4.0X10-1 | 1.1X101 | 3.0X103 | 8.2X104 |
Sn-119m | | 4.0X101 | 1.1X103 | 3.0X101 | 8.1X102 | 1.4X102 | 3.7X103 |
Sn-121m (a) | | 4.0X101 | 1.1X103 | 9.0X10-1 | 2.4X101 | 2.0 | 5.4X101 |
Sn-123 | | 8.0X10-1 | 2.2X101 | 6.0X10-1 | 1.6X101 | 3.0X102 | 8.2X103 |
Sn-125 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 4.0X103 | 1.1X105 |
Sn-126 (a) | | 6.0X10-1 | 1.6X101 | 4.0X10-1 | 1.1X101 | 1.0X10-3 | 2.8X10-2 |
Sr-82 (a) | Strontium (38) | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 2.3X103 | 6.2X104 |
Sr-85 | | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 8.8X102 | 2.4X104 |
Sr-85m | | 5.0 | 1.4X102 | 5.0 | 1.4X102 | 1.2X106 | 3.3X107 |
Sr-87m | | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 4.8X105 | 1.3X107 |
Sr-89 | | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 1.1X103 | 2.9X104 |
Sr-90 (a) | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 5.1 | 1.4X102 |
Sr-91 (a) | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.3X105 | 3.6X106 |
Sr-92 (a) | | 1.0 | 2.7X101 | 3.0X10-1 | 8.1 | 4.7X105 | 1.3X107 |
T(H-3) | Tritium (1) | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 3.6X102 | 9.7X103 |
Ta-178 (long-lived) | Tantalum (73) | 1.0 | 2.7X101 | 8.0X10-1 | 2.2X101 | 4.2X106 | 1.1X108 |
Ta-179 | | 3.0X101 | 8.1X102 | 3.0X101 | 8.1X102 | 4.1X101 | 1.1X103 |
Ta-182 | | 9.0X10-1 | 2.4X101 | 5.0X10-1 | 1.4X101 | 2.3X102 | 6.2X103 |
Tb-157 | Terbium (65) | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 5.6X10-1 | 1.5X101 |
Tb-158 | | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 5.6X10-1 | 1.5X101 |
Tb-160 | | 1.0 | 2.7X101 | 6.0X10-1 | 1.6X101 | 4.2X102 | 1.1X104 |
Tc-95m (a) | Technetium (43) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 8.3X102 | 2.2X104 |
Tc-96 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 1.2X104 | 3.2X105 |
Tc-96m (a) | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 1.4X106 | 3.8X107 |
Tc-97 | | Unlimited | Unlimited | Unlimited | Unlimited | 5.2X10-5 | 1.4X10-3 |
Tc-97m | | 4.0X101 | 1.1X103 | 1.0 | 2.7X101 | 5.6X102 | 1.5X104 |
Tc-98 | | 8.0X10-1 | 2.2X101 | 7.0X10-1 | 1.9X101 | 3.2X10-5 | 8.7X10-4 |
Tc-99 | | 4.0X101 | 1.1X103 | 9.0X10-1 | 2.4X101 | 6.3X10-4 | 1.7X10-2 |
Tc-99m | | 1.0X101 | 2.7X102 | 4.0 | 1.1X102 | 1.9X105 | 5.3X106 |
Te-121 | Tellurium (52) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 2.4X103 | 6.4X104 |
Te-121m | | 5.0 | 1.4X102 | 3.0 | 8.1X101 | 2.6X102 | 7.0X103 |
Te-123m | | 8.0 | 2.2X102 | 1.0 | 2.7X101 | 3.3X102 | 8.9X103 |
Te-125m | | 2.0X101 | 5.4X102 | 9.0X10-1 | 2.4X101 | 6.7X102 | 1.8X104 |
Te-127 | | 2.0X101 | 5.4X102 | 7.0X10-1 | 1.9X101 | 9.8X104 | 2.6X106 |
Te-127m (a) | | 2.0X101 | 5.4X102 | 5.0X10-1 | 1.4X101 | 3.5X102 | 9.4X103 |
Te-129 | | 7.0X10-1 | 1.9X101 | 6.0X10-1 | 1.6X101 | 7.7X105 | 2.1X107 |
Te-129m (a) | | 8.0X10-1 | 2.2X101 | 4.0X10-1 | 1.1X101 | 1.1X103 | 3.0X104 |
Te-131m (a) | | 7.0X10-1 | 1.9X101 | 5.0X10-1 | 1.4X101 | 3.0X104 | 8.0X105 |
Te-132 (a) | | 5.0X10-1 | 1.4X101 | 4.0X10-1 | 1.1X101 | 1.1X104 | 3.0X105 |
Th-227 | Thorium (90) | 1.0X101 | 2.7X102 | 5.0X10-3 | 1.4X10-1 | 1.1X103 | 3.1X104 |
Th-228 (a) | | 5.0X10-1 | 1.4X101 | 1.0X10-3 | 2.7X10-2 | 3.0X101 | 8.2X102 |
Th-229 | | 5.0 | 1.4X102 | 5.0X10-4 | 1.4X10-2 | 7.9X10-3 | 2.1X10-1 |
Th-230 | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 7.6X10-4 | 2.1X10-2 |
Th-231 | | 4.0X101 | 1.1X103 | 2.0X10-2 | 5.4X10-1 | 2.0X104 | 5.3X105 |
Th-232 | | Unlimited | Unlimited | Unlimited | Unlimited | 4.0X10-9 | 1.1X10-7 |
Th-234 (a) | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 8.6X102 | 2.3X104 |
Th(nat) | | Unlimited | Unlimited | Unlimited | Unlimited | 8.1X10-9 | 2.2X10-7 |
Ti-44 (a) | Titanium (22) | 5.0X10-1 | 1.4X101 | 4.0X10-1 | 1.1X101 | 6.4 | 1.7X102 |
Tl-200 | Thallium (81) | 9.0X10-1 | 2.4X101 | 9.0X10-1 | 2.4X101 | 2.2X104 | 6.0X105 |
Tl-201 | | 1.0X101 | 2.7X102 | 4.0 | 1.1X102 | 7.9X103 | 2.1X105 |
Tl-202 | | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 2.0X103 | 5.3X104 |
Tl-204 | | 1.0X101 | 2.7X102 | 7.0X10-1 | 1.9X101 | 1.7X101 | 4.6X102 |
Tm-167 | Thulium (69) | 7.0 | 1.9X102 | 8.0X10-1 | 2.2X101 | 3.1X103 | 8.5X104 |
Tm-170 | | 3.0 | 8.1X101 | 6.0X10-1 | 1.6X101 | 2.2X102 | 6.0X103 |
Tm-171 | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 |
U-230 (fast lung absorption) (a)(d) | Uranium (92) | 4.0X101 | 1.1X103 | 1.0X10-1 | 2.7 | 1.0X103 | 2.7X104 |
U-230 (medium lungabsorption) (a)(e) | | 4.0X101 | 1.1X103 | 4.0X10-3 | 1.1X10-1 | 1.0X103 | 2.7X104 |
U-230 (slow lung absorption) (a)(f) | | 3.0X101 | 8.1X102 | 3.0X10-3 | 8.1X10-2 | 1.0X103 | 2.7X104 |
U-232 (fast lung absorption) (d) | | 4.0X101 | 1.1X103 | 1.0X10-2 | 2.7X10-1 | 8.3X10-1 | 2.2X101 |
U-232 (medium lung absorption) (e) | | 4.0X101 | 1.1X103 | 7.0X10-3 | 1.9X10-1 | 8.3X10-1 | 2.2X101 |
U-232 (slow lung absorption) (f) | | 1.0X101 | 2.7X102 | 1.0X10-3 | 2.7X10-2 | 8.3X10-1 | 2.2X101 |
U-233 (fast lung absorption) (d) | | 4.0X101 | 1.1X103 | 9.0X10-2 | 2.4 | 3.6X10-4 | 9.7X10-3 |
U-233 (medium lung absorption) (e) | | 4.0X101 | 1.1X103 | 2.0X10-2 | 5.4X10-1 | 3.6X10-4 | 9.7X10-3 |
U-233 (slow lung absorption) (f) | | 4.0X101 | 1.1X103 | 6.0X10-3 | 1.6X10-1 | 3.6X10-4 | 9.7X10-3 |
U-234 (fast lung absorption) (d) | | 4.0X101 | 1.1X103 | 9.0X10-2 | 2.4 | 2.3X10-4 | 6.2X10-3 |
U-234 (medium lung absorption) (e) | | 4.0X101 | 1.1X103 | 2.0X10-2 | 5.4X10-1 | 2.3X10-4 | 6.2X10-3 |
U-234 (slow lung absorption)(f) | | 4.0X101 | 1.1X103 | 6.0X10-3 | 1.6X10-1 | 2.3X10-4 | 6.2X10-3 |
U-235 (all lung absorption types) (a),(d),(e),(f) | | Unlimited | Unlimited | Unlimited | Unlimited | 8.0X10-8 | 2.2X10-6 |
U-236 (fast lung absorption) (d) | | Unlimited | Unlimited | Unlimited | Unlimited | 2.4X10-6 | 6.5X10-5 |
U-236 (medium lung absorption) (e) | | 4.0X101 | 1.1X103 | 2.0X10-2 | 5.4X10-1 | 2.4X10-6 | 6.5X10-5 |
U-236 (slow lung absorption) (f) | | 4.0X101 | 1.1X103 | 6.0X10-3 | 1.6X10-1 | 2.4X10-6 | 6.5X10-5 |
U-238 (all lung absorption types) (d),(e),(f) | | Unlimited | Unlimited | Unlimited | Unlimited | 1.2X10-8 | 3.4X10-7 |
U (nat) | | Unlimited | Unlimited | Unlimited | Unlimited | 2.6X10-8 | 7.1X10-7 |
U (enriched to 20% or less) (g) | | Unlimited | Unlimited | Unlimited | Unlimited | See Table
A-4 | See Table
A-4 |
U (dep) | | Unlimited | Unlimited | Unlimited | Unlimited | See Table
A-4 | See Table
A-3 |
V-48 | Vanadium (23) | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 6.3X103 | 1.7X105 |
V-49 | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 3.0X102 | 8.1X103 |
W-178 (a) | Tungsten (74) | 9.0 | 2.4X102 | 5.0 | 1.4X102 | 1.3X103 | 3.4X104 |
W-181 | | 3.0X101 | 8.1X102 | 3.0X101 | 8.1X102 | 2.2X102 | 6.0X103 |
W-185 | | 4.0X101 | 1.1X103 | 8.0X10-1 | 2.2X101 | 3.5X102 | 9.4X103 |
W-187 | | 2.0 | 5.4X101 | 6.0X10-1 | 1.6X101 | 2.6X104 | 7.0X105 |
W-188 (a) | | 4.0X10-1 | 1.1X101 | 3.0X10-1 | 8.1 | 3.7X102 | 1.0X104 |
Xe-122 (a) | Xenon (54) | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 4.8X104 | 1.3X106 |
Xe-123 | | 2.0 | 5.4X101 | 7.0X10-1 | 1.9X101 | 4.4X105 | 1.2X10 |
Xe-127 | | 4.0 | 1.1X102 | 2.0 | 5.4X101 | 1.0X103 | 2.8X104 |
Xe-131m | | 4.0X101 | 1.1X103 | 4.0X101 | 1.1X103 | 3.1X103 | 8.4X104 |
Xe-133 | | 2.0X101 | 5.4X102 | 1.0X101 | 2.7X102 | 6.9X103 | 1.9X105 |
Xe-135 | | 3.0 | 8.1X101 | 2.0 | 5.4X101 | 9.5X104 | 2.6X106 |
Y-87 (a) | Yttrium (39) | 1.0 | 2.7X101 | 1.0 | 2.7X101 | 1.7X104 | 4.5X105 |
Y-88 | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 5.2X102 | 1.4X104 |
Y-90 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 2.0X104 | 5.4X105 |
Y-91 | | 6.0X10-1 | 1.6X101 | 6.0X10-1 | 1.6X101 | 9.1X102 | 2.5X104 |
Y-91m | | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 1.5X106 | 4.2X10 |
Y-92 | | 2.0X10-1 | 5.4 | 2.0X10-1 | 5.4 | 3.6X105 | 9.6X106 |
Y-93 | | 3.0X10-1 | 8.1 | 3.0X10-1 | 8.1 | 1.2X105 | 3.3X106 |
Yb-169 | Ytterbium (70) | 4.0 | 1.1X102 | 1.0 | 2.7X101 | 8.9X102 | 2.4X104 |
Yb-175 | | 3.0X101 | 8.1X102 | 9.0X10-1 | 2.4X101 | 6.6X103 | 1.8X105 |
Zn-65 | Zinc (30) | 2.0 | 5.4X101 | 2.0 | 5.4X101 | 3.0X102 | 8.2X103 |
Zn-69 | | 3.0 | 8.1X101 | 6.0X10-1 | 1.6X101 | 1.8X106 | 4.9X10 |
Zn-69m (a) | | 3.0 | 8.1X101 | 6.0X10-1 | 1.6X101 | 1.2X105 | 3.3X106 |
Zr-88 | Zirconium (40) | 3.0 | 8.1X101 | 3.0 | 8.1X101 | 6.6X102 | 1.8X104 |
Zr-93 | | Unlimited | Unlimited | Unlimited | Unlimited | 9.3X10 | 2.5X10 |
Zr-95 (a) | | 2.0 | 5.4X101 | 8.0X10-1 | 2.2X101 | 7.9X102 | 2.1X104 |
Zr-97 (a) | | 4.0X10-1 | 1.1X101 | 4.0X10-1 | 1.1X101 | 7.1X104 | 1.9X106 |
aA1 and/or A2 values include contributions from daughter nuclides with half-lives less than 10 days., as listed in the following: |
Mg-28
Ca-47
Ti-44
Fe-52
Fe-60
Zn-69m
Ge-68
Rb-83
Sr-82
Sr-90
Sr-91
Sr-92
Y-87
Zr-95
Zr-97
Mo-99
Tc-95m
Tc-96m
Ru-103
Ru-106
Pd-103
Ag-108m
Ag-110m
Cd-115
In-114m
Sn-113
Sn-121m
Sn-126
Te-127m
Te-129m
Te-131m
Te-132
I-135
Xe-122
Cs-137
Ba-131
Ba-140
Ce-144
Pm-148m
Gd-146
Dy-166
Hf-172
W-178
W-188
Re-189
Os-194
Ir-189
Pt-188
Hg-194
Hg-195m
Pb-210
Pb-212
Bi-210m
Bi-212
At-211
Rn-222
Ra-223
Ra-224
Ra-225
Ra-228
Ac-225
Ac-227
Th-228
Th-234
Pa-230
U-230
U-235
Pu-241
Pu-244
Am-242m
Am-243
Cm-247
Bk-249
Cf-253 | Al-28
Sc-47
Sc-44
Mn-52m
Co-60m
Zn-69
Ga-68
Kr-83m
Rb-82
Y-90
Y-91m
Y-92
Sr-87m
Nb-95m
Nb-97m, Nb-97
Tc-99m
Tc-95
Tc-96
Rh-103m
Rh-106
Rh-103m
Ag-108
Ag-110
In-115m
In-114
In-113m
Sn-121
Sb-126m
Te-127
Te-129
Te-131
I-132
Xe-135m
I-122
Ba-137m
Cs-131
La-140
Pr-144m, Pr-144
Pm-148
Eu-146
Ho-166
Lu-172
Ta-178
Re-188
Os-189m
Ir-194
Os-189m
Ir-188
Au-194
Hg-195
Bi-210
Bi-212, Po-212, TI-208
Tl-206
Tl-208, Po-212
Po-211
Po-218, Pb-214, At-218, Bi-214, Po-214
Rn-219, Po-215, Pb-211, Bi-211, Po-211, Tl-207
Rn-220, Po-216, Pb-212, Bi-212, Tl-208, Po-212
Ac-225, Fr-221, At-217, Bi-213, Tl-209, Po-213, Pb-209
Ac-228
Fr-221, At-217, Bi-213, Po-213, Pb-209, TI-209
Fr-223
Ra-224, Rn-220, Po-216, Pb-212, Bi-212, Tl-208, Po-212
Pa-234m, Pa-234
Ac-226, Th-226, Fr-222, Ra-222, Rn-218, Po-214
Th-226, Ra-222, Rn-218, Po-214
Th-231
U-237
U-240, Np-240m
Am-242, Np-238
Np-239
Pu-243
Am-245
Cm-249 |
bThe values of A1 and A2 in Curies (Ci) are approximate and for information only; the regulatory standard units are terabecquerels (TBq).
cThe quantity activity of Ir-192 in special form may be determined from a measurement of the rate of decay or a measurement of the radiation level at a prescribed distance from the source.
dThese values apply only to compounds of uranium that take the chemical form of UF6, UO2F2 and UO2(NO3)2 in both normal and accident conditions of transport.
eThese values apply only to compounds of uranium that take the chemical form of UO3, UF4, UCl4 and hexavalent compounds in both normal and accident conditions of transport.
fThese values apply to all compounds of uranium other than those specified in notes d and e of this table.
gThese values apply to unirradiated uranium only.
hA1 = 0.1 TBq (2.7 Ci) and A2 = 0.001 TBq (0.027 Ci) for Cf-252 for domestic use.
I h A2 = 0.74 TBq (20 Ci) for Mo-99 for domestic use. |
| | | | | | | | |
G. Table 2. Exempt Material Activity Concentrations and Exempt Consignment Activity Limits for Radionuclides.
Symbol of radionuclide | Element and atomic number | Activity concentration for exempt material (Bq/g) | Activity concentration for exempt material (Ci/g) | Activity limit for exempt consignment (Bq) | Activity limit for exempt consignment (Ci) |
Ac-225 | Actinium (89) | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Ac-227 | | 1.0X10-1 | 2.7X10-12 | 1.0X103 | 2.7X10-8 |
Ac-228 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Ag-105 | Silver (47) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ag-108m (b) | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Ag-110m | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Ag-111 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Al-26 | Aluminum (13) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Am-241 | Americium (95) | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Am-242m (b) | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Am-243 (b) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Ar-37 | Argon (18) | 1.0X106 | 2.7X10-5 | 1.0X108 | 2.7X10-3 |
Ar-39 | | 1.0X107 | 2.7X10-4 | 1.0X104 | 2.7X10-7 |
Ar-41 | | 1.0X102 | 2.7X10-9 | 1.0X109 | 2.7X10-2 |
As-72 | Arsenic (33) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
As-73 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
As-74 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
As-76 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
As-77 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
At-211 | Astatine (85) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Au-193 | Gold (79) | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Au-194 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Au-195 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Au-198 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Au-199 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ba-131 | Barium (56) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ba-133 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ba-133m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ba-140 (b) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Be-7 | Beryllium (4) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Be-10 | | 1.0X104 | 2.7X10-7 | 1.0X106 | 2.7X10-5 |
Bi-205 | Bismuth (83) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Bi-206 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Bi-207 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Bi-210 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Bi-210m | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Bi-212 (b) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Bk-247 | Berkelium (97) | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Bk-249 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Br-76 | Bromine (35) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Br-77 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Br-82 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
C-11 | Carbon (6) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
C-14 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Ca-41 | Calcium (20) | 1.0X105 | 2.7X10-6 | 1.0X107 | 2.7X10-4 |
Ca-45 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Ca-47 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Cd-109 | Cadmium (48) | 1.0X104 | 2.7X10-7 | 1.0X106 | 2.7X10-5 |
Cd-113m | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Cd-115 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Cd-115m | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Ce-139 | Cerium (58) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ce-141 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Ce-143 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ce-144 (b) | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Cf-248 | Californium (98) | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Cf-249 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Cf-250 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Cf-251 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Cf-252 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Cf-253 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Cf-254 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Cl-36 | Chlorine (17) | 1.0X104 | 2.7X10-7 | 1.0X106 | 2.7X10-5 |
Cl-38 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Cm-240 | Curium (96) | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Cm-241 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Cm-242 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Cm-243 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Cm-244 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Cm-245 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Cm-246 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Cm-247 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Cm-248 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Co-55 | Cobalt (27) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Co-56 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Co-57 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Co-58 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Co-58m | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Co-60 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Cr-51 | Chromium (24) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Cs-129 | Cesium (55) | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Cs-131 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Cs-132 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Cs-134 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Cs-134m | | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
Cs-135 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Cs-136 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Cs-137 (b) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Cu-64 | Copper (29) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Cu-67 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Dy-159 | Dysprosium (66) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Dy-165 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Dy-166 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Er-169 | Erbium (68) | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Er-171 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Eu-147 | Europium (63) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Eu-148 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Eu-149 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Eu-150 (short lived) | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Eu-150 (long lived) | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Eu-152 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Eu-152m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Eu-154 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Eu-155 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Eu-156 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
F-18 | Fluorine (9) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Fe-52 | Iron (26) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Fe-55 | | 1.0X104 | 2.7X10-7 | 1.0X106 | 2.7X10-5 |
Fe-59 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Fe-60 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Ga-67 | Gallium (31) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ga-68 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Ga-72 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Gd-146 | Gadolinium (64) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Gd-148 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Gd-153 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Gd-159 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Ge-68 | Germanium (32) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Ge-71 | | 1.0X104 | 2.7X10-7 | 1.0X108 | 2.7X10-3 |
Ge-77 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Hf-172 | Hafnium (72) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Hf-175 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Hf-181 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Hf-182 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Hg-194 | Mercury (80) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Hg-195m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Hg-197 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Hg-197m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Hg-203 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Ho-166 | Holmium (67) | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
Ho-166m | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
I-123 | Iodine (53) | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
I-124 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
I-125 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
I-126 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
I-129 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
I-131 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
I-132 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
I-133 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
I-134 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
I-135 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
In-111 | Indium (49) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
In-113m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
In-114m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
In-115m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ir-189 | Iridium (77) | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Ir-190 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Ir-192 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Ir-194 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
K-40 | Potassium (19) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
K-42 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
K-43 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Kr-79 | Krypton (36) | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
Kr-81 | Krypton (36)
| 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Kr-85 | | 1.0X105 | 2.7X10-6 | 1.0X104 | 2.7X10-7 |
Kr-85m | | 1.0X103 | 2.7X10-8 | 1.0X1010 | 2.7X10-1 |
Kr-87 | | 1.0X102 | 2.7X10-9 | 1.0X109 | 2.7X10-2 |
La-137 | Lanthanum (57) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
La-140 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Lu-172 | Lutetium (71) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Lu-173 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Lu-174 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Lu-174m | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Lu-177 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Mg-28 | Magnesium (12) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Mn-52 | Manganese (25) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Mn-53 | | 1.0X104 | 2.7X10-7 | 1.0X109 | 2.7X10-2 |
Mn-54 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Mn-56 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Mo-93 | Molybdenum (42) | 1.0X103 | 2.7X10-8 | 1.0X108 | 2.7X10-3 |
Mo-99 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
N-13 | Nitrogen (7) | 1.0X102 | 2.7X10-9 | 1.0X109 | 2.7X10-2 |
Na-22 | Sodium (11) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Na-24 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Nb-93m | Niobium (41) | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Nb-94 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Nb-95 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Nb-97 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Nd-147 | Neodymium (60) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Nd-149 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ni-59 | Nickel (28) | 1.0X104 | 2.7X10-7 | 1.0X108 | 2.7X10-3 |
Ni-63 | | 1.0X105 | 2.7X10-6 | 1.0X108 | 2.7X10-3 |
Ni-65 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Np-235 | Neptunium (93) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Np-236 (short-lived) | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Np-236 (long-lived) | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Np-237 (b) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Np-239 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Os-185 | Osmium (76) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Os-191 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Os-191m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Os-193 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Os-194 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
P-32 | Phosphorus (15) | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
P-33 | | 1.0X105 | 2.7X10-6 | 1.0X108 | 2.7X10-3 |
Pa-230 | Protactinium (91) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Pa-231 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Pa-233 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Pb-201 | Lead (82) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Pb-202 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Pb-203 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Pb-205 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Pb-210 (b) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Pb-212 (b) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Pd-103 | Palladium (46) | 1.0X103 | 2.7X10-8 | 1.0X108 | 2.7X10-3 |
Pd-107 | | 1.0X105 | 2.7X10-6 | 1.0X108 | 2.7X10-3 |
Pd-109 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Pm-143 | Promethium (61) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Pm-144 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Pm-145 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Pm-147 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Pm-148m | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Pm-149 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Pm-151 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Po-210 | Polonium (84) | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Pr-142 | Praseodymium (59) | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Pr-143 | | 1.0X104 | 2.7X10-7 | 1.0X106 | 2.7X10-5 |
Pt-188 | Platinum (78) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Pt-191 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Pt-193 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Pt-193m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Pt-195m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Pt-197 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Pt-197m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Pu-236 | Plutonium (94) | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Pu-237 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Pu-238 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Pu-239 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Pu-240 | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Pu-241 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Pu-242 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Pu-244 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Ra-223 (b) | Radium (88) | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Ra-224 (b) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Ra-225 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Ra-226 (b) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Ra-228 (b) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Rb-81 | Rubidium (37) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Rb-83 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Rb-84 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Rb-86 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Rb-87 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Rb(nat) | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Re-184 | Rhenium (75) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Re-184m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Re-186 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Re-187 | | 1.0X106 | 2.7X10-5 | 1.0X109 | 2.7X10-2 |
Re-188 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Re-189 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Re(nat) | | 1.0X106 | 2.7X10-5 | 1.0X109 | 2.7X10-2 |
Rh-99 | Rhodium (45) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Rh-101 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Rh-102 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Rh-102m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Rh-103m | | 1.0X104 | 2.7X10-7 | 1.0X108 | 2.7X10-3 |
Rh-105 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Rn-222 (b) | Radon (86) | 1.0X101 | 2.7X10-10 | 1.0X108 | 2.7X10-3 |
Ru-97 | Ruthenium (44) | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Ru-103 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Ru-105 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Ru-106 (b) | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
S-35 | Sulphur (16) | 1.0X105 | 2.7X10-6 | 1.0X108 | 2.7X10-3 |
Sb-122 | Antimony (51) | 1.0X102 | 2.7X10-9 | 1.0X104 | 2.7X10-7 |
Sb-124 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Sb-125 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Sb-126 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Sc-44 | Scandium (21) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Sc-46 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Sc-47 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Sc-48 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Se-75 | Selenium (34) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Se-79 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Si-31 | Silicon (14) | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Si-32 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Sm-145 | Samarium (62) | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Sm-147 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Sm-151 | | 1.0X104 | 2.7X10-7 | 1.0X108 | 2.7X10-3 |
Sm-153 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Sn-113 | Tin (50) | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Sn-117m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Sn-119m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Sn-121m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Sn-123 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Sn-125 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Sn-126 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Sr-82 | Strontium (38) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Sr-85 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Sr-85m | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Sr-87m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Sr-89 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Sr-90 (b) | | 1.0X102 | 2.7X10-9 | 1.0X104 | 2.7X10-7 |
Sr-91 | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Sr-92 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
T(H-3) | Tritium (1) | 1.0X106 | 2.7X10-5 | 1.0X109 | 2.7X10-2 |
Ta-178 (long-lived) | Tantalum (73) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Ta-179 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Ta-182 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Tb-157 | Terbium (65) | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Tb-158 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Tb-160 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Tc-95m | Technetium (43) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Tc-96 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Tc-96m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Tc-97 | | 1.0X103 | 2.7X10-8 | 1.0X108 | 2.7X10-3 |
Tc-97m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Tc-98 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Tc-99 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
Tc-99m | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Te-121 | Tellurium (52) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Te-121m | | 1.0X102 | 2.7X10-9 | 1.0X1065 | 2.7X10-65 |
Te-123m | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Te-125m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Te-127 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Te-127m | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Te-129 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Te-129m | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Te-131m | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Te-132 | | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Th-227 | Thorium (90) | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Th-228 (b) | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Th-229 (b) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Th-230 | | 1.0 | 2.7X10-11 | 1.0X104 | 2.7X10-7 |
Th-231 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Th-232 | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
Th-234 (b) | | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
Th (nat) (b) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
Ti-44 | Titanium (22) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
Tl-200 | Thallium (81) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Tl-201 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Tl-202 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Tl-204 | | 1.0X104 | 2.7X10-7 | 1.0X104 | 2.7X10-7 |
Tm-167 | Thulium (69) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Tm-170 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Tm-171 | | 1.0X104 | 2.7X10-7 | 1.0X108 | 2.7X10-3 |
U-230 (fast lung absorption) (b),(d) | Uranium (92) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
U-230 (medium lung absorption) (e) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-230 (slow lung absorption) (f) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-232 (fast lung absorption) (b),(d) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
U-232 (medium lung absorption) (e) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-232 (slow lung absorption) (f) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-233 (fast lung absorption) (d) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-233 (medium lung absorption) (e) | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
U-233 (slow lung absorption) (f) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
U-234 (fast lung absorption) (d) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-234 (medium lung absorption) (e) | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
U-234 (slow lung absorption) (f) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
U-235 (all lung absorption types) (b),(d),(e),(f) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-236 (fast lung absorption) (d) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-236 (medium lung absorption) (e) | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
U-236 (slow lung absorption) (f) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U-238 (all lung absorption types) (b),(d),(e),(f) | | 1.0X101 | 2.7X10-10 | 1.0X104 | 2.7X10-7 |
U (nat) (b) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
U (enriched to 20% or less) (g) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
U (dep) | | 1.0 | 2.7X10-11 | 1.0X103 | 2.7X10-8 |
V-48 | Vanadium (23) | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
V-49 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
W-178 | Tungsten (74) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
W-181 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
W-185 | | 1.0X104 | 2.7X10-7 | 1.0X107 | 2.7X10-4 |
W-187 | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
W-188 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Xe-122 | Xenon (54) | 1.0X102 | 2.7X10-9 | 1.0X109 | 2.7X10-2 |
Xe-123 | | 1.0X102 | 2.7X10-9 | 1.0X109 | 2.7X10-2 |
Xe-127 | | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
Xe-131m | | 1.0X104 | 2.7X10-7 | 1.0X104 | 2.7X10-7 |
Xe-133 | | 1.0X103 | 2.7X10-8 | 1.0X104 | 2.7X10-7 |
Xe-135 | | 1.0X103 | 2.7X10-8 | 1.0X1010 | 2.7X10-1 |
Y-87 | Yttrium (39) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Y-88 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Y-90 | | 1.0X103 | 2.7X10-8 | 1.0X105 | 2.7X10-6 |
Y-91 | | 1.0X103 | 2.7X10-8 | 1.0X106 | 2.7X10-5 |
Y-91m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Y-92 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Y-93 | | 1.0X102 | 2.7X10-9 | 1.0X105 | 2.7X10-6 |
Yb-169 | Ytterbium (70) | 1.0X102 | 2.7X10-9 | 1.0X107 | 2.7X10-4 |
Yb-175 | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Zn-65 | Zinc (30) | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Zn-69 | | 1.0X104 | 2.7X10-7 | 1.0X106 | 2.7X10-5 |
Zn-69m | | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Zr-88 | Zirconium (40) | 1.0X102 | 2.7X10-9 | 1.0X106 | 2.7X10-5 |
Zr-93 (b) | | 1.0X103 | 2.7X10-8 | 1.0X107 | 2.7X10-4 |
Zr-95 | | 1.0X101 | 2.7X10-10 | 1.0X106 | 2.7X10-5 |
Zr-97 (b) | | 1.0X101 | 2.7X10-10 | 1.0X105 | 2.7X10-6 |
a(Reserved)
bParent nuclides and their progeny included in secular equilibrium are listed in the following: |
Sr-90 | Y-90 |
Zr-93 | Nb-93m |
Zr-97 | Nb-97 |
Ru-106 | Rh-106 |
Cs-137 | Ba-137m |
Ce-134
| La-134
|
Ce-144 | Pr-144 |
Ba-140 | La-140 |
Bi-212 | Tl-208 (0.36), Po-212 (0.64) |
Pb-210 | Bi-210, Po-210 |
Pb-212 | Bi-212, Tl-208 (0.36), Po-212 (0.64) |
Rn-220
| Po-216
|
Rn-222 | Po-218, Pb-214, Bi-214, Po-214 |
Ra-223 | Rn-219, Po-215, Pb-211, Bi-211, Tl-207 |
Ra-224 | Rn-220, Po-216, Pb-212, Bi-212, Tl-208(0.36), Po-212 (0.64) |
Ra-226 | Rn-222, Po-218, Pb-214, Bi-214, Po-214, Pb-210, Bi-210, Po-210 |
Ra-228 | Ac-228 |
Th-226
| Ra-222, Rn-218, Po-214
|
Th-228 | Ra-224, Rn-220, Po-216, Pb-212, Bi-212, Tl-208 (0.36), Po-212 (0.64) |
Th-229 | Ra-225, Ac-225, Fr-221, At-217, Bi-213, Po-213, Pb-209 |
Th-nat | Ra-228, Ac-228, Th-228, Ra-224, Rn-220, Po-216, Pb-212, Bi-212, Tl-208 (0.36), Po-212 (0.64) |
Th-234 | Pa-234m |
U-230 | Th-226, Ra-222, Rn-218, Po-214 |
U-232 | Th-228, Ra-224, Rn-220, Po-216, Pb-212, Bi-212, Tl-208 (0.36), Po-212 (0.64) |
U-235 | Th-231 |
U-238 | Th-234, Pa-234m |
U-nat | Th-234, Pa-234m, U-234, Th-230, Ra-226, Rn-222, Po-218, Pb-214, Bi-214, Po-214, Pb-210, Bi-210, Po-210 |
U-240
| Np-240m
|
Np-237 | Pa-233 |
Am-242m | Am-242 |
Am-243 | Np-239 |
c(Reserved)
dThese values apply only to compounds of uranium that take the chemical form of UF6, UO2F2 and UO2(NO3)2 in both normal and accident conditions of transport.
eThese values apply only to compounds of uranium that take the chemical form of UO3, UF4, UCl4 and hexavalent compounds in both normal and accident conditions of transport.
fThese values apply to all compounds of uranium other than those specified in notes (d) and (e) of this table.
gThese values apply to unirradiated uranium only. |
H. Table 3. General Values for A1 and A2.
Contents | A1 | A2 | Activity concentration for exempt material (Bq/g) | Activity concentration for exempt material (Ci/g) | Activity limits for exempt consignments (Bq) | Activity limits for exempt consignments (Ci) |
(TBq) | (Ci) | (TBq) | (Ci) |
Only beta or gamma emitting radionuclides are known to be present | 1 x 10-1 | 2.7 x 100 | 2 x 10-2 | 5.4 x 10-1 | 1 x 101 | 2.7 x10-10 | 1 x 104 | 2.7 x10-7 |
Only alpha Alpha emitting radionuclides, but no neutron emitters, are known to be present (a)
| 2 x 10-1 | 5.4 x 100 | 9 x 10-5 | 2.4 x 10-3 | 1 x 10-1 | 2.7 x10-12 | 1 x 103 | 2.7 x10-8 |
No relevant data are available Neutron emitting nuclides are known to be present or no relevant data are available
| 1 x 10-3 | 2.7 x 10-2 | 9 x 10-5 | 2.4 x 10-3 | 1 x 10-1 | 2.7 x 10-12 | 1 x 103 | 2.7 x10-8 |
aIf beta or gamma emitting nuclides are known to be present, the A1 value of 0.1 TBq (2.7 Ci) should be used. |
I. Table 4. Activity-Mass Relationships for Uranium.
Uranium Enrichment1
wt % U-235 present | Specific Activity |
TBq/g | Ci/g |
0.45 | 1.8 x 10-8 | 5.0 x 10-7 |
0.72 | 2.6 x 10-8 | 7.1 x 10-7 |
1 | 2.8 x 10-8 | 7.6 x 10-7 |
1.5 | 3.7 x 10-8 | 1.0 x 10-6 |
5 | 1.0 x 10-7 | 2.7 x 10-6 |
10 | 1.8 x 10-7 | 4.8 x 10-6 |
20 | 3.7 x 10-7 | 1.0 x 10-5 |
35 | 7.4 x 10-7 | 2.0 x 10-5 |
50 | 9.3 x 10-7 | 2.5 x 10-5 |
90 | 2.2 x 10-6 | 5.8 x 10-5 |
93 | 2.6 x 10-6 | 7.0 x 10-5 |
95 | 3.4 x 10-6 | 9.1 x 10-5 |
1The figures for uranium include representative values for the activity of the uranium-234 that is concentrated during the enrichment process. |
VA.R. Doc. No. R18-5063; Filed August 29, 2017, 5:04 p.m.
TITLE 13. HOUSING
BOARD OF HOUSING AND COMMUNITY DEVELOPMENT
Final Regulation
REGISTRAR'S NOTICE: The
Board of Housing and Community Development is claiming an exemption from
Article 2 of the Administrative Process Act in accordance with § 2.2-4006
A 4 a of the Code of Virginia, which excludes regulations that are necessary to
conform to changes in Virginia statutory law where no agency discretion is
involved. The Board of Housing and Community Development will receive,
consider, and respond to petitions by any interested person at any time with
respect to reconsideration or revision.
Title of Regulation:
13VAC5-112. Enterprise Zone Grant Program Regulation (amending 13VAC5-112-10).
Statutory Authority: § 59.1-541 of the Code of Virginia.
Effective Date: January 1, 2018.
Agency Contact: Elizabeth O. Rafferty, Policy and
Legislative Director, Department of Housing and Community Development, Main
Street Centre, 600 East Main Street, Suite 300, Richmond, VA 23219, telephone
(804) 371-7011, FAX (804) 371-7090, TTY (804) 371-7089, or email
elizabeth.rafferty@dhcd.virginia.gov.
Summary:
The amendments conform to Chapter 451 of the 2017 Acts of
Assembly by providing that an expenditure for an improvement to real property
may qualify for a grant or tax credit regardless of whether it is capitalized
or deducted as a business expense under federal Treasury Regulations.
Part I
Definitions
13VAC5-112-10. Definitions.
The following words and terms when used in this chapter shall
have the following meanings unless the context clearly indicates otherwise:
"Agreed-upon procedures engagement" means an
engagement between an independent certified public accountant licensed by the
Commonwealth and the business or zone investor seeking to qualify for
Enterprise Zone incentive grants pursuant to § 59.1-549 of the Code of
Virginia whereby the independent certified public accountant, using procedures
specified by the department, will test and report on the assertion of the
business or zone investor as to their qualification to receive the Enterprise
Zone incentive.
"Assumption or acquisition" means, in connection
with a trade or business, that the inventory, accounts receivable, liabilities,
customer list and good will of an existing Virginia company has been assumed or
acquired by another taxpayer, regardless of a change in federal identification
number or employees.
"Average number of permanent full-time employees"
means the number of permanent full-time employees during each payroll period of
a business firm's taxable year divided by the number of payroll periods. This
definition applies only for the purpose of qualifying for Enterprise Zone
incentives pursuant to 13VAC5-112-20:
1. In calculating the average number of permanent full-time
employees, a business firm may count only those permanent full-time employees
who worked at least half of their normal workdays during the payroll period.
Paid leave time may be counted as work time.
2. For a business firm that uses different payroll periods for
different classes of employees, the average number of permanent full-time
employees of the firm shall be defined as the sum of the average number of
permanent full-time employees for each class of employee.
"Base taxable year" means either of two taxable
years immediately preceding the first year of qualification, at the choice of
the business firm. This definition applies only for the purpose of qualifying
for Enterprise Zone incentives pursuant to 13VAC5-112-20.
"Base year" means either of the two calendar years
immediately preceding a qualified business firm's first year of grant
eligibility, at the choice of the business firm.
"Building" means any construction meeting the
common ordinarily accepted meaning of the term (building, a usually roofed and
walled structure built for permanent use) where (i) areas separated by interior
floors or other horizontal assemblies and (ii) areas separated by fire walls or
vertical assemblies shall not be construed to constitute separate buildings,
irrespective of having separate addresses, ownership or tax assessment
configurations, unless there is a property line contiguous with the fire wall or
vertical assembly.
"Business firm" means any corporation, partnership,
electing small business (subchapter S) corporation, limited liability company,
or sole proprietorship authorized to do business in the Commonwealth of
Virginia. This shall also include business and professional organizations and
associations whose classification falls under sectors 813910 and 813920 of the
North American Industry Classification Systems and that generate the majority
of their revenue from customers outside the Commonwealth.
"Capital lease" means a lease that meets one or
more of the following criteria and as such is classified as a purchase by the
lessee: the lease term is greater than 75% of the property's estimated economic
life; the lease contains an option to purchase the property for less than fair
market value; ownership of the property is transferred to the lessee at the end
of the lease term; or the present value of the lease payments exceed 90% of the
fair market value of the property.
"Common control" means those firms as defined by
Internal Revenue Code § 52(b).
"Department" means the Department of Housing and
Community Development.
"Establishment" means a single physical location
where business is conducted or where services or industrial operations are performed.
1. A central administrative office is an establishment
primarily engaged in management and general administrative functions performed
centrally for other establishments of the same firm.
2. An auxiliary unit is an establishment primarily engaged in
performing supporting services to other establishments of the same firm. This
definition applies only for the purpose of qualifying for Enterprise Zone
incentives pursuant to 13VAC5-112-110.
"Existing business firm" means one that was
actively engaged in the conduct of trade or business in an area prior to such
an area being designated as an enterprise zone or that was engaged in the
conduct of trade or business in the Commonwealth and relocates to begin
operation of a trade or business within an enterprise zone. An existing
business firm is also one that was not previously conducted in the Commonwealth
by such taxpayer who acquires or assumes a trade or business and continues its
operations. This definition applies only for the purpose of qualifying for
Enterprise Zone incentives pursuant to 13VAC5-112-20.
"Expansion" means an increase in square footage or
the footprint of an existing nonresidential building via a shared wall, or
enlargement of an existing room or floor plan. Pursuant to real property investment
grants this shall include mixed-use buildings.
"Facility" means a complex of buildings, co-located
at a single physical location within an enterprise zone, all of which are
necessary to facilitate the conduct of the same trade or business. This
definition applies to new construction, as well as to the rehabilitation and
expansion of existing structures.
"Federal minimum wage" means the minimum wage
standard as currently defined by the United States U.S.
Department of Labor in the Fair Labor Standards Act, 29 USC § 201 et seq. Such
definition applies to permanent full-time employees paid on an hourly or wage
basis.
"Food and beverage service" means a business whose
classification falls under subsector 722 Food Services and Drinking Places of
North American Industry Classification System.
"Full month" means the number of days that a
permanent full-time position must be filled in order to count in the
calculation of the grant amount under 13VAC5-112-260. A full month is
calculated by dividing the total number of days in calendar year by 12. A full
month for the purpose of calculating job creation grants is equivalent to
30.416666 days.
"Grant-eligible position" means a new permanent
full-time position created above the threshold number at an eligible business
firm. Positions in retail, personal service or food and beverage service shall
not be considered grant-eligible positions.
"Health benefits" means that at a minimum medical
insurance is offered to employees, and the employer shall offer to pay
at least 50% of the cost of the premium at the time of employment and annually
thereafter.
"High unemployment area" means enterprise zone
localities with unemployment rates one and one-half times or more than the
state average based on the most recent annualized unemployment data published
by the Virginia Employment Commission.
"Household" means all the persons who occupy a
single housing unit. Occupants may be a single family, one person living alone,
two or more families living together, or any group of related or unrelated
persons who share living arrangements. This definition applies only for the
purpose of qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-20.
"Household income" means all income actually
received by all household members over the age of 16 years from the
following sources. This definition applies only for the purpose of qualifying
for Enterprise Zone incentives pursuant to 13VAC5-112-20:
1. Gross wages, salaries, tips, commissions, etc. (before
deductions);
2. Net self-employment income (gross receipts minus operating
expenses);
3. Interest and dividend earnings; and
4. Other money income received from net rents, Old Age and
Survivors Insurance, social security benefits, pensions, alimony, child
support, and periodic income from insurance policy annuities and other sources.
The following types of income are excluded from household
income:
1. Noncash benefits such as food stamps and housing
assistance;
2. Public assistance payments;
3. Disability payments;
4. Unemployment and employment training benefits;
5. Capital gains and losses; and
6. One-time unearned income.
When computing household income, income of a household member
shall be counted for the portion of the income determination period that the
person was actually a part of the household.
"Household size" means the largest number of
household members during the income determination period. This definition
applies only for the purpose of qualifying for Enterprise Zone incentives
pursuant to 13VAC5-112-20.
"Housing unit" means a house, apartment, group of
rooms, or single room that is occupied or intended for occupancy as separate
living quarters. This definition applies only for the purpose of qualifying for
Enterprise Zone incentives pursuant to 13VAC5-112-20.
"Income determination period" means the 12 months
immediately preceding the month in which the person was hired. This definition
applies only for the purpose of qualifying for Enterprise Zone incentives
pursuant to 13VAC5-112-20.
"Independent certified public accountant" means a
public accountant certified and licensed by the Commonwealth of Virginia who is
not an employee of the business firm seeking to qualify for state tax
incentives and grants under this program.
"Job creation grant" means a grant provided under § 59.1-547
of the Code of Virginia.
"Joint enterprise zone" means an enterprise zone located
in two or more adjacent localities.
"Jurisdiction" means the city or county which
that made the application to have an enterprise zone. In the case of a
joint application, it means all parties making the application. Pursuant to
enterprise zone designations made prior to July 1, 2005, this shall include
towns.
"Large qualified business firm" means a qualified
business firm making qualified zone investments in excess of $15 million when
such zone investments result in the creation of at least 50 permanent full-time
positions. This definition applies only for the purpose of qualifying for
Enterprise Zone incentives pursuant to 13VAC5-112-20.
"Large qualified zone resident" means a qualified
zone resident making qualified zone investments in excess of $100 million when
such qualified zone investments result in the creation of at least 200
permanent full-time positions. This definition applies only for the purpose of
qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-110.
"Local zone administrator" means the chief
executive of the city or county, in which an enterprise zone is located, or his
designee. Pursuant to enterprise zone designations made prior to July 1, 2005,
this shall include towns.
"Low-income" means household income was less than
or equal to 80% of area median household income during the income determination
period. Persons who meet the definition of both low-income and zone resident
may not be counted as both for purposes of meeting employment requirements for
the general tax credit. Instead, qualifying business firms must claim these
persons as either low-income or zone resident. This definition applies only for
the purpose of qualifying for Enterprise Zone incentives pursuant to
13VAC5-112-20.
"Median household income" means the dollar amount,
adjusted for household size, as determined annually by the department for the
city or county in which the zone is located. This definition applies only for
the purpose of qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-20.
"Mixed use" means a building incorporating
residential uses in which a minimum of 30% of the useable floor space will be
devoted to commercial, office or industrial use. Buildings where less than 30%
of the useable floor space is devoted to commercial, office or industrial use
shall be considered primarily residential in nature and shall not be eligible
for a grant under 13VAC5-112-330. This definition applies only for the purpose
of qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-330.
"Net loss" applies to firms that relocate or expand
operations and means (i) after relocating into a zone, a business firm's gross
permanent employment is less than it was before locating into the zone, or (ii)
after a business firm locates or expands within a zone, its gross employment at
its nonzone location or locations is less than it was before the zone location
occurred.
"New business" means a business not previously
conducted in the Commonwealth by such taxpayer and that begins operation in an
enterprise zone after the zone was designated. A new business is also one
created by the establishment of a new facility and new permanent full-time
employment by an existing business firm in an enterprise zone and does not
result in a net loss of permanent full-time employment outside the zone. This
definition applies only for the purpose of qualifying for Enterprise Zone
incentives pursuant to 13VAC5-112-20.
"New construction" means a single, nonresidential
facility built on previously undeveloped land of a nonresidential structure
built on the site/parcel site or parcel of a previously razed
structure with no remnants of the prior structure or physical connection to
existing structures or outbuildings on the property. Pursuant to real property
investment grants this shall include mixed-use buildings.
"Number of eligible permanent full-time positions"
means the amount by which the number of permanent full-time positions at a
business firm in a grant year exceeds the threshold number. This definition
applies only for the purpose of qualifying for Enterprise Zone incentives
pursuant to 13VAC5-112-260.
"Payroll period" means the period of time for which
a business firm normally pays its employees.
"Permanent full-time employee" means a person
employed by a business firm who is normally scheduled to work either (i)
a minimum of 35 hours per week for the entire normal year of the business
firm's operations, which normal year must consist of at least 48 weeks, (ii) a
minimum of 35 hours per week for a portion of the taxable year in which the
employee was initially hired for, or transferred to the business firm, or (iii)
a minimum of 1,680 hours per year if the standard fringe benefits are paid by
the business firm for the employee. Permanent full-time employee also means two
or more individuals who together share the same job position and together work
the normal number of hours a week as required by the business firm for that one
position. Seasonal, temporary, leased or contract labor employees or employees
shifted from an existing location in the Commonwealth to a business firm
location within an enterprise zone shall not qualify as permanent full-time
employees. This definition only applies to business firms for the purpose of
qualifying for enterprise zone incentives pursuant to 13VAC5-112-20.
"Permanent full-time position" (for the purpose of
qualifying for grants pursuant to § 59.1-547 of the Code of Virginia) means a
job of indefinite duration at a business firm located within an enterprise zone
requiring the employee to report to work within the enterprise zone; and requiring
(i) a minimum of 35 hours of an employee's time per week for the entire normal
year of the business firm's operation, which "normal year" must
consist of at least 48 weeks, (ii) a minimum of 35 hours of an employee's time
per week for the portion of the calendar year in which the employee was
initially hired for or transferred to the business firm, or (iii) a minimum of
1,680 hours per year. Such position shall not include (a) seasonal, temporary
or contract positions, (b) a position created when a job function is shifted
from an existing location in the Commonwealth to a business firm located with
an enterprise zone, (c) any position that previously existed in the
Commonwealth, or (d) positions created by a business that is simultaneously
closing facilities in other areas of the Commonwealth.
"Personal service" means such positions classified
under NAICS 812.
"Placed in service" means the final certificate of
occupancy has been issued or the final building inspection has been approved by
the local jurisdiction for real property improvements or real property
investments, or in cases where a project does not require permits, the licensed
third party inspector's report that the project was complete; or pursuant to
13VAC5-112-110 the first moment that machinery becomes operational and is used
in the manufacturing of a product for consumption; or in the case of tools and
equipment, the first moment they are used in the performance of duty or
service.
"Qualification year" the calendar year for which a
qualified business firm or qualified zone investor is applying for a grant
pursuant to 13VAC5-112-260.
"Qualified business firm" means a business firm
meeting the business firm requirements in 13VAC5-112-20 or 13VAC5-112-260 and
designated a qualified business firm by the department.
"Qualified real property investment" (for purposes
of qualifying for a real property investment grant) means the amount properly
chargeable to a capital account expended for improvements to
rehabilitate, expand, or construct depreciable real property placed in
service during the calendar year within an enterprise zone provided that the
total amount of such improvements equals or exceeds (i) $100,000 with respect
to a single building or a facility in the case of rehabilitation or expansion
or (ii) $500,000 with respect to a single building or a facility in the case of
new construction. "Qualified real property investment" includes
any such expenditure regardless of whether it is considered properly chargeable
to a capital account or deductible as a business expense under federal Treasury
regulations. Qualified real property investments include expenditures
associated with (a) any exterior, interior, structural, mechanical or
electrical improvements necessary to construct, expand or rehabilitate a
building for commercial, industrial or mixed use; (b) excavations; (c) grading
and paving; (d) installing driveways; and (e) landscaping or land improvements.
Qualified real property investments shall include, but not be limited to, costs
associated with demolition, carpentry, sheetrock, plaster, painting, ceilings,
fixtures, doors, windows, fire suppression systems, roofing, flashing, exterior
repair, cleaning and cleanup.
Qualified real property investment shall not include:
1. The cost of acquiring any real property or building.
2. Other costs including (i) the cost of furnishings; (ii) any
expenditure associated with appraisal, architectural, engineering, surveying,
and interior design fees; (iii) loan fees, points, or capitalized interest;
(iv) legal, accounting, realtor, sales and marketing, or other professional
fees; (v) closing costs, permits, user fees, zoning fees, impact fees, and
inspection fees; (vi) bids, insurance, signage, utilities, bonding, copying,
rent loss, or temporary facilities incurred during construction; (vii) utility
connection or access fees; (viii) outbuildings; (ix) the cost of any well or
septic or sewer system; and (x) roads.
3. The basis of any property (i) for which a grant under this
section was previously provided; (ii) for which a tax credit under § 59.1-280.1
of the Code of Virginia was previously granted; (iii) that was previously
placed in service in Virginia by the qualified zone investor, a related party
as defined by Internal Revenue Code § 267(b), or a trade or business under
common control as defined by Internal Revenue Code § 52(b); or (iv) that was
previously in service in Virginia and has a basis in the hands of the person
acquiring it, determined in whole or in part by reference to the basis of such
property in the hands of the person from whom it was acquired or Internal
Revenue Code § 1014(a).
"Qualified zone improvements" (for purposes of
qualifying for an Investment Tax Credit) means the amount properly
chargeable to a capital account expended for improvements to
rehabilitate or expand depreciable nonresidential real property placed in
service during the taxable year within an enterprise zone, provided that the
total amount of such improvements equals or exceeds (i) $50,000 and (ii) the
assessed value of the original facility immediately prior to the rehabilitation
or expansion. "Qualified zone expenditures" includes any such
expenditure regardless of whether it is considered properly chargeable to a
capital account or deductible as a business expense under federal Treasury
regulations. Qualified zone improvements include expenditures associated
with any exterior, structural, mechanical, or electrical improvements necessary
to construct, expand or rehabilitate a building for commercial or industrial
use.
1. Qualified zone improvements include, but are not limited
to, the costs associated with excavation, grading, paving, driveways,
roads, sidewalks, landscaping or other land improvements, demolition,
carpentry, sheetrock, plaster, painting, ceilings, fixtures, doors, windows,
fire suppression systems, roofing and flashing, exterior repair, cleaning and
clean-up.
2. Qualified zone improvements do not include (i) the cost of
furnishings; (ii) any expenditure associated with appraisal, architectural,
engineering and interior design fees; (iii) loan fees, points or capitalized
interest; (iv) legal, accounting, realtor, sales and marketing or other
professional fees; (v) closing costs, permits, user fees, zoning fees, impact
fees, inspection fees; (vi) bids insurance, signage, utilities, bonding,
copying, rent loss, or temporary facilities incurred during construction; (vii)
utility hook-up or access fees; (viii) outbuildings; (ix) the cost of any well,
septic, or sewer system; or (x) cost of acquiring land or an existing building.
3. In the case of new nonresidential construction, qualified
zone improvements also do not include land, land improvements, paving, grading,
driveway, and interest. This definition applies only for the purposes of
qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-110.
"Qualified zone investment" means the sum of
qualified zone improvements and the cost of machinery, tools and equipment used
in manufacturing tangible personal property and placed in service on or after
July 1, 1995. Machinery, equipment, tools, and real property that are leased
through a capital lease and that are being depreciated by the lessee or that
are transferred from out-of-state to a zone location by a business firm may be
included as qualified zone investment. Such leased or transferred machinery,
equipment, tools, and real property shall be valued using the depreciable basis
for federal income tax purposes. Machinery, tools and equipment shall not
include the basis of any property: (i) for which a credit was previously
granted under § 59.1-280.1 of the Code of Virginia; (ii) that was previously
placed in service in Virginia by the taxpayer, a related party, as defined by
Internal Revenue Code § 267(b), or a trade or business under common control, as
defined by Internal Revenue Code § 52(b); or (iii) that was previously in
service in Virginia and has a basis in the hands of the person acquiring it,
determined in whole or in part by reference to the basis of such property in the
hands of the person whom acquired it, or Internal Revenue Code § 1014(a). This
definition applies only for the purposes of qualifying for Enterprise Zone
incentives pursuant to 13VAC5-112-110.
"Qualified zone investor" means an owner or tenant
of real property located within an enterprise zone who expands, rehabilitates
or constructs such real property for commercial, industrial or mixed use. In
the case of a tenant, the amounts of qualified zone investment specified in
this section shall relate to the proportion of the building or facility for
which the tenant holds a valid lease. In the case of an owner of an individual
unit within a horizontal property regime, the amounts of qualified zone
investments specified in this section shall relate to that proportion of the
building for which the owner holds title and not to common elements.
Units of local, state and federal government or political subdivisions
shall not be considered qualified zone investors.
"Qualified zone resident" means an owner or tenant
of nonresidential real property located in an enterprise zone who expands or
rehabilitates such real property to facilitate the conduct of a trade or
business by such owner or tenant within the enterprise zone. In the case of a
partnership, limited liability company or S corporation, the term
"qualified zone resident" means the partnership, limited liability
company or S corporation. This definition applies only for the purposes of
qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-110.
"Real property investment grant" means a grant made
under § 59.1-548 of the Code of Virginia. This definition applies only for
the purposes of qualifying for Enterprise Zone incentives pursuant to
13VAC5-112-330.
"Reduced wage rate threshold" means 150% of the
federal minimum wage pursuant to 13VAC5-112-270, 13VAC5-112-280, and
13VAC5-112-285 and high unemployment areas.
"Rehabilitation" means the alteration or renovation
of all or part of an existing nonresidential building without an increase in
square footage. Pursuant to real property investment grants this shall include
mixed-use buildings.
"Regular basis" means at least once a month. This
definition applies only for the purposes of qualifying for Enterprise Zone
incentives pursuant to 13VAC5-112-260.
"Related party" means those as defined by Internal
Revenue Code § 267(b).
"Report to work" means that the employee filling a
permanent full-time position reports to the business' zone establishment on a
regular basis.
"Retail" means a business whose classification
falls under sectors 44-45 Retail Trade of North American Industry
Classification System.
"Same trade or business" means the operations of a
single company or related companies or companies under common control.
"Seasonal employee" means any employee who normally
works on a full-time basis and whose customary annual employment is less than
nine months. For example, individuals hired by a CPA firm during the tax return
season in order to process returns and who work full-time over a three month period
are seasonal employees.
"Small qualified business firm" means any qualified
business firm other than a large qualified business firm. This definition
applies only for the purpose of qualifying for Enterprise Zone incentives
pursuant to 13VAC5-112-20.
"Small qualified zone resident" means any qualified
zone resident other than a large qualified zone resident. This definition
applies only for the purpose of qualifying for Enterprise Zone incentives
pursuant to 13VAC5-112-350 C.
"Subsequent base year" means the base year for
calculating the number of grant-eligible positions in a second or subsequent
five consecutive calendar year grant period. If a second or subsequent
five-year grant period is requested within two years after the previous
five-year grant period, the subsequent base year will be the last grant year.
The calculation of this subsequent base year employment will be determined by
the number of permanent full-time positions in the preceding base year, plus
the number of threshold positions, plus the number of grant-eligible positions
in the final year of the previous grant period. If a business firm applies for
subsequent five consecutive calendar-year grant periods beyond the two years
immediately following the completion of the previous five-year grant period,
the business firm shall use one of the two preceding calendar years as
subsequent base year, at the choice of the business firm.
"Tax due" means the amount of tax liability as
determined by the Department of Taxation or the State Corporation Commission.
This definition applies only for the purpose of qualifying for Enterprise Zone
incentives pursuant to 13VAC5-112-20 and 13VAC5-112-110.
"Tax year" means the year in which the assessment
is made. This definition applies only for the purpose of qualifying for
Enterprise Zone incentives pursuant to 13VAC5-112-110.
"Taxable year" means the year in which the tax due
on state taxable income, state taxable gross receipts, or state taxable
net capital is accrued. This definition applies only for the purpose of
qualifying for Enterprise Zone incentives pursuant to 13VAC5-112-20 and
13VAC5-112-110.
"Threshold number" means an increase of four
permanent full-time positions over the number of permanent full-time positions
in the base year or subsequent base year.
"Transferred employee" means an employee of a firm
in the Commonwealth that is relocated to an enterprise zone facility owned or
operated by that firm.
"Useable floor space" means all space in a building
finished as appropriate to the use(s) use of the building as
represented in measured drawings. Unfinished basements, attics, and parking
garages would not constitute useable floor space. Finished common areas such as
stairwells and elevator shafts should be apportioned appropriately based on the
majority use (51%) of that floor(s) floor.
"Wage rate" means the hourly wage paid to an
employee inclusive of shift premiums and commissions. In the case of salaried
employees, the hourly wage rate shall be determined by dividing the annual salary,
inclusive of shift premiums and commissions, by 1,820 hours. Bonuses, overtime
and tips are not to be included in the determination of wage rate.
"Zone" means an enterprise zone declared by the
Governor to be eligible for the benefits of this program.
"Zone real property investment tax credit" means a
credit provided to a large qualified zone resident pursuant to § 59.1-280.1 J
of the Code of Virginia. This definition applies only for qualifying for
Enterprise Zone incentives pursuant to 13VAC5-112-110.
"Zone resident" means a person whose principal
place of residency is within the boundaries of any enterprise zone. Persons who
meet the definition of both low-income and zone resident may not be counted as
both for purposes of meeting employment requirements for the general tax
credit. Instead, qualifying business firms must claim these persons as either
low-income or zone resident. Zone residency must be verified annually. This
definition applies only for qualifying for Enterprise Zone incentives pursuant
to 13VAC5-112-20.
VA.R. Doc. No. R18-5157; Filed August 30, 2017, 8:26 a.m.
TITLE 14. INSURANCE
STATE CORPORATION COMMISSION
Final Regulation
REGISTRAR'S NOTICE: The State Corporation Commission is claiming an exemption from the Administrative Process Act in accordance with § 2.2-4002 A 2 of the Code of Virginia, which exempts courts, any agency of the Supreme Court, and any agency that by the Constitution is expressly granted any of the powers of a court of record.
Title of Regulation: 14VAC5-170. Rules Governing Minimum Standards for Medicare Supplement Policies (amending 14VAC5-170-30, 14VAC5-170-60, 14VAC5-170-85, 14VAC5-170-150; adding 14VAC5-170-87).
Statutory Authority: §§ 12.1-13 and 38.2-223 of the Code of Virginia.
Effective Date: October 1, 2017.
Agency Contact: James Young, Policy Advisor, Policy and Compliance Division, Bureau of Insurance, State Corporation Commission, 1300 East Main Street, 6th Floor, Richmond, VA 23219, mailing address: P.O. Box 1157, Richmond, VA 23218, telephone (804) 371-9612, FAX (804) 371994, or email james.young@scc.virginia.gov.
Summary:
The amendments (i) conform the regulations to the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), which prohibits the sale of Medigap policies that cover Part B deductibles to newly eligible Medicare beneficiaries, defined as those individuals who have attained age 65 years on or after January 1, 2020, or first become eligible for Medicare due to age, disability, or end-stage renal disease on or after January 1, 2020, and (ii) update deductible amounts.
AT RICHMOND, AUGUST 21, 2017
COMMONWEALTH OF VIRGINIA, ex rel.
STATE CORPORATION COMMISSION
CASE NO. INS-2017-00141
Ex Parte: In the matter of Amending
the Rules Governing Minimum Standards
for Medicare Supplement Policies
ORDER ADOPTING REVISIONS TO RULES
On June 20, 2017, the State Corporation Commission ("Commission") issued an Order to Take Notice ("Order") to consider revisions to the Rules Governing Minimum Standards for Medicare Supplement Policies set forth in Chapter 170 of Title 14 of the Virginia Administrative Code ("Rules").
These amendments were proposed by the Bureau of Insurance ("Bureau") to conform the Rules to the Medicare Access and CHIP Reauthorization Act of 2015 ("MACRA"), which was signed into law on April 16, 2015. This piece of legislation prohibits the sale of Medigap policies that cover Part B deductibles to "newly eligible" Medicare beneficiaries, defined as those individuals who have attained age 65 on or after January 1, 2020, or first become eligible for Medicare due to age, disability, or end-stage renal disease on or after January 1, 2020. In addition to the changes made pursuant to MACRA, the proposed amendments include updated deductible amounts.
The Order required that on or before August 10, 2017, any person requesting a hearing on the amendments to the Rules shall have filed such request for a hearing with the Clerk of the Commission ("Clerk"). No request for a hearing was filed with the Clerk.
The Order also required any interested persons to file with the Clerk their comments in support of or in opposition to the amendments to the Rules on or before August 10, 2017. No comments were filed with the Clerk.
NOW THE COMMISSION, having considered the proposed amendments to the Rules, is of the opinion that the attached amendments to the Rules should be adopted.
Accordingly, IT IS ORDERED THAT:
(1) The amendments to the Rules Governing Minimum Standards for Medicare Supplement Policies at Chapter 170 of Title 14 of the Virginia Administrative Code, which amend the Rules at 14 VAC 5-170-30, 14 VAC 5-170-60, 14 VAC 5-170-85, and 14 VAC 5-170-150, and add a new Rule at 14 VAC 5-170-87, and which are attached hereto and made a part hereof, are hereby ADOPTED, to be effective October 1, 2017.
(2) The Bureau forthwith shall give notice of the adoption of the amendments to the Rules to all health insurance issuers licensed to issue policies of accident and sickness insurance, subscription contracts, or evidences of coverage in this Commonwealth and to all interested persons.
(3) The Commission's Division of Information Resources forthwith shall cause a copy of this Order, together with the final amended Rules, to be forwarded to the Virginia Registrar of Regulations for appropriate publication in the Virginia Register of Regulations.
(4) The Commission's Division of Information Resources shall make available this Order and the attached amendments to the Rules on the Commission's website: http://www.scc.virginia.gov/case.
(5) The Bureau shall file with the Clerk of the Commission an affidavit of compliance with the notice requirements of Ordering Paragraph (2) above.
(6) This case is dismissed, and the papers herein shall be placed in the file for ended causes.
AN ATTESTED COPY hereof shall be sent by the Clerk of the Commission to: Kiva B. Pierce, Assistant Attorney General, Division of Consumer Counsel, Office of the Attorney General, 202 N. 9th Street, 8th Floor, Richmond, Virginia 23219-3424; and a copy hereof shall be delivered to the Commission's Office of General Counsel and the Bureau of Insurance in care of Deputy Commissioner Donald Beatty.
14VAC5-170-30. Definitions.
The following words and terms when used in this chapter shall have the following meanings unless the context clearly indicates otherwise:
"1990 standardized Medicare supplement benefit plan," "1990 standardized benefit plan" or "1990 plan" means a group or individual policy of Medicare supplement insurance issued on or after July 30, 1992, and with an effective date for coverage prior to June 1, 2010, and includes Medicare supplement insurance policies and certificates renewed on or after that date that are not replaced by the issuer at the request of the insured.
"2010 standardized Medicare supplement benefit plan," "2010 standardized benefit plan" or "2010 plan" means a group or individual policy of Medicare supplement insurance issued with an effective date for coverage on or after June 1, 2010.
"Applicant" means:
1. In the case of an individual Medicare supplement policy, the person who seeks to contract for insurance benefits; and
2. In the case of a group Medicare supplement policy, the proposed certificateholder.
"Attained age rating" means a premium structure under which premiums are based on the covered individual's age at the time of application of the policy or certificate, and for which premiums increase based on the covered individual's increase in age during the life of the policy or certificate.
"Bankruptcy" means when a Medicare Advantage organization that is not an issuer has filed, or has had filed against it, a petition for declaration of bankruptcy and has ceased doing business in this Commonwealth.
"Certificate" means any certificate delivered or issued for delivery in this Commonwealth under a group Medicare supplement policy.
"Certificate form" means the form on which the certificate is delivered or issued for delivery by the issuer.
"Community rating" means a premium structure under which premium rates are the same for all covered individuals of all ages in a given area.
"Continuous period of creditable coverage" means the period during which an individual was covered by creditable coverage, if during the period of the coverage the individual did not have a break in coverage greater than 63 days.
"Creditable coverage" means, with respect to an individual, coverage of the individual provided under any of the following:
1. A group health plan;
2. Health insurance coverage;
3. Part A or Part B of Title XVIII of the Social Security Act of 1935 (Medicare) (42 USC § 1395 et seq.);
4. Title XIX of the Social Security Act of 1935 (Medicaid) (42 USC § 1396 et seq.), other than coverage consisting solely of benefits under § 1928;
5. Chapter 55 of Title 10 of the United States Code (CHAMPUS) (TRICARE) (10 USC§§ 1071-1107);
6. A medical care program of the Indian Health Service or of a tribal organization;
7. A state health benefits risk pool;
8. A health plan offered under the Federal Employees Health Benefits Act of 1959 (5 USC §§ 8901-8914);
9. A public health plan as defined in federal regulation; and
10. A health benefit plan under § 5(e) of the Peace Corps Act of 1961 (22 USC § 2504(e)).
"Creditable coverage" shall not include one or more, or any combination of, the following:
1. Coverage only for accident or disability income insurance, or any combination thereof;
2. Coverage issued as a supplement to liability insurance;
3. Liability insurance, including general liability insurance and automobile liability insurance;
4. Workers' compensation or similar insurance;
5. Automobile medical expense insurance;
6. Credit-only insurance;
7. Coverage for on-site medical clinics; and
8. Other similar insurance coverage, specified in federal regulations, under which benefits for medical care are secondary or incidental to other insurance benefits.
"Creditable coverage" shall not include the following benefits if they are provided under a separate policy, certificate or contract of insurance or are otherwise not an integral part of the plan:
1. Limited scope dental or vision benefits;
2. Benefits for long-term care, nursing home care, home health care, community-based care or any combination thereof; and
3. Such other similar, limited benefits as are specified in federal regulations.
"Creditable coverage" shall not include the following benefits if offered as independent, noncoordinated benefits:
1. Coverage only for a specified disease or illness; and
2. Hospital indemnity or other fixed indemnity insurance.
"Creditable coverage" shall not include the following if it is offered as a separate policy, certificate or contract of insurance:
1. Medicare supplement health insurance as defined under § 1882(g)(1) of the Social Security Act of 1935 (42 USC § 1395ss);
2. Coverage supplemental to the coverage provided under Chapter 55 of Title 10 of the United States Code (10 USC §§ 1071-1107); and
3. Similar supplemental coverage provided to coverage under a group health plan.
"Employee welfare benefit plan" means a plan, fund or program of employee benefits as defined in the Employee Retirement Income Security Act of 1974 (29 USC § 1002).
"Insolvency" means when an issuer, duly licensed to transact an insurance business in this Commonwealth in accordance with the provisions of Chapter 10, 41, 42 or 43, respectively, of Title 38.2 of the Code of Virginia, is determined to be insolvent and placed under a final order of liquidation by a court of competent jurisdiction.
"Issue age rating" means a premium structure based upon the covered individual's age at the time of purchase of the policy or certificate. Under an issue age rating structure, premiums do not increase due to the covered individual's increase in age during the life of the policy or certificate.
"Issuer" includes insurance companies, fraternal benefit societies, corporations licensed pursuant to Chapter 42 of Title 38.2 of the Code of Virginia to offer health services plans, health maintenance organizations, and any other entity delivering or issuing for delivery in this Commonwealth Medicare supplement policies or certificates.
"Medicare" means the "Health Insurance for the Aged Act," Title XVIII of the Social Security Act (42 USC § 1395 et seq.), as then constituted or later amended.
"Medicare Advantage plan" means a plan of coverage for health benefits under Medicare Part C as defined in § 1859 (42 USC § 1395w-28(b)(1) of the Social Security Act, and includes:
1. Coordinated care plans which that provide health care services, including but not limited to health maintenance organization plans (with or without a point-of-service option), plans offered by provider-sponsored organizations, and preferred provider organization plans;
2. Medical savings account plans coupled with a contribution into a Medicare Advantage medical savings account; and
3. Medicare Advantage private fee-for-service plans.
"Medicare supplement policy" means a group or individual policy of accident and sickness insurance or a subscriber contract of health service plans or health maintenance organizations, other than a policy issued pursuant to a contract under § 1876 of the federal Social Security Act of 1935 (42 USC § 1395 et seq.) or an issued policy under a demonstration project specified in 42 USC § 1395ss(g)(1), which is advertised, marketed or designed primarily as a supplement to reimbursements under Medicare for the hospital, medical or surgical expenses of persons eligible for Medicare. "Medicare supplement policy" does not include Medicare Advantage plans established under Medicare Part C, Outpatient Prescription Drug plans established under Medicare Part D, or any Health Care Prepayment Plan that provides benefits pursuant to an agreement under § 1833(a)(1)(A) of the Social Security Act.
"Policy form" means the form on which the policy is delivered or issued for delivery by the issuer.
"Prestandardized Medicare supplement benefit plan," "prestandardized benefit plan" or "prestandardized plan" means a group or individual policy of Medicare supplement insurance issued prior to July 30, 1992.
"Secretary" means the Secretary of the United States U.S. Department of Health and Human Services.
14VAC5-170-60. Minimum benefit standards for prestandardized Medicare supplement benefits plan policies or certificates issued for delivery prior to July 30, 1992.
A. No policy or certificate may be advertised, solicited or issued for delivery in this Commonwealth as a Medicare supplement policy or certificate unless it meets or exceeds the following minimum standards. These are minimum standards and do not preclude the inclusion of other provisions or benefits which that are not inconsistent with these standards.
B. The following standards apply to Medicare supplement policies and certificates and are in addition to all other requirements of this chapter.
1. A Medicare supplement policy or certificate shall not exclude or limit benefits for a loss incurred more than six months from the effective date of coverage because it involved a preexisting condition. The policy or certificate shall not define a preexisting condition more restrictively than a condition for which medical advice was given or treatment was recommended by or received from a physician within six months before the effective date of coverage.
2. A Medicare supplement policy or certificate shall not indemnify against losses resulting from sickness on a different basis than losses resulting from accidents.
3. A Medicare supplement policy or certificate shall provide that benefits designed to cover cost sharing amounts under Medicare will be changed automatically to coincide with any changes in the applicable Medicare deductible, copayment or coinsurance amounts. Premiums may be modified to correspond with such changes.
4. A "noncancellable," "guaranteed renewable," or "noncancellable and guaranteed renewable" Medicare supplement policy shall not:
a. Provide for termination of coverage of a spouse solely because of the occurrence of an event specified for termination of coverage of the insured, other than the nonpayment of premium; or
b. Be cancelled canceled or nonrenewed by the issuer solely on the grounds of deterioration of health.
5. a. Except as authorized by the State Corporation Commission, an issuer shall neither cancel nor nonrenew a Medicare supplement policy or certificate for any reason other than nonpayment of premium or material misrepresentation.
b. If a group Medicare supplement insurance policy is terminated by the group policyholder and not replaced as provided in subdivision 5 d of this subsection, the issuer shall offer certificateholders an individual Medicare supplement policy. The issuer shall offer the certificateholder at least the following choices:
(1) An individual Medicare supplement policy currently offered by the issuer having comparable benefits to those contained in the terminated group Medicare supplement policy; and
(2) An individual Medicare supplement policy which that provides only such benefits as are required to meet the minimum standards as defined in 14VAC5-170-75 C.
c. If membership in a group is terminated, the issuer shall:
(1) Offer the certificateholder the conversion opportunities described in subdivision 5 b of this subsection; or
(2) At the option of the group policyholder, offer the certificateholder continuation of coverage under the group policy.
d. If a group Medicare supplement policy is replaced by another group Medicare supplement policy purchased by the same policyholder, the issuer of the replacement policy shall offer coverage to all persons covered under the old group policy on its date of termination. Coverage under the new group policy shall not result in any exclusion for preexisting conditions that would have been covered under the group policy being replaced.
6. Termination of a Medicare supplement policy or certificate shall be without prejudice to any continuous loss which commenced while the policy was in force, but the extension of benefits beyond the period during which the policy was in force may be predicated upon the continuous total disability of the insured, limited to the duration of the policy benefit period, if any, or to payment of the maximum benefits. Receipt of Medicare Part D benefits will not be considered in determining a continuous loss.
7. If a Medicare supplement policy is modified to eliminate an outpatient prescription drug benefit as a result of requirements imposed by the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (42 USC § 1395w-101), the modified policy shall be deemed to satisfy the guaranteed renewal requirements of this subsection.
C. Minimum benefit standards.
1. Coverage of Part A Medicare eligible expenses for hospitalization to the extent not covered by Medicare from the 61st day through the 90th day in any Medicare benefit period;
2. Coverage for either all or none of the Medicare Part A inpatient hospital deductible amount;
3. Coverage of Part A Medicare eligible expenses incurred as daily hospital charges during use of Medicare's lifetime hospital inpatient reserve days;
4. Upon exhaustion of all Medicare hospital inpatient coverage including the lifetime reserve days, coverage of 90% of all Medicare Part A eligible expenses for hospitalization not covered by Medicare subject to a lifetime maximum benefit of an additional 365 days;
5. Coverage under Medicare Part A for the reasonable cost of the first three pints of blood (or equivalent quantities of packed red blood cells, as defined under federal regulations) unless replaced in accordance with federal regulations or already paid for under Part B;
6. Coverage for the coinsurance amount, or in the case of hospital outpatient department services paid under a prospective payment system, the copayment amount of Medicare eligible expenses under Part B regardless of hospital confinement, subject to a maximum calendar year out-of-pocket amount equal to the Medicare Part B deductible $100 established by the Centers for Medicare and Medicaid Services;
7. Effective January 1, 1990, coverage under Medicare Part B for the reasonable cost of the first three pints of blood (or equivalent quantities of packed red blood cells, as defined under federal regulations), unless replaced in accordance with federal regulations or already paid for under Part A, subject to the Medicare deductible amount.
14VAC5-170-85. Standard plans for 2010 standardized Medicare supplement policies delivered on or after June 1, 2010.
A. The following standard plans are applicable to all Medicare supplement benefit plan policies or certificates delivered or issued for delivery in this Commonwealth with an effective date for coverage on or after June 1, 2010. No policy or certificate may be advertised, solicited, delivered or issued for delivery in this Commonwealth as a Medicare supplement policy or certificate unless it complies with these benefit plan standards. Benefit plan standards applicable to Medicare supplement policies and certificates issued with an effective date for coverage before June 1, 2010, remain subject to the requirements of 14VAC5-170-80.
B. 1. An issuer shall make available to each prospective policyholder and certificateholder a policy form or certificate form containing only the basic (core) benefits, as defined in 14VAC5-170-75 C.
2. If an issuer makes available any of the additional benefits described in 14VAC5-170-75 D, or offers standardized benefit Plans K or L (as described in subdivisions F 8 and F 9 of this section), then the issuer shall make available to each prospective policyholder and certificateholder, in addition to a policy form or certificate form with only the basic (core) benefits as described in subdivision 1 of this subsection, a policy form or certificate form containing either standardized benefit Plan C (as described in subdivision F 3 of this section) or standardized benefit Plan F (as described in subdivision F 5 of this section).
C. No groups, packages or combinations of Medicare supplement benefits other than those listed in this section shall be offered for sale in this Commonwealth, except as may be permitted in subsection G of this section and 14VAC5-170-90.
D. Benefit plans shall be uniform in structure, language, designation and format to the standard benefit plans listed in this subsection and conform to the definitions in 14VAC5-170-30. Each benefit shall be structured in accordance with the format provided in 14VAC5-170-75 C and D; or, in the case of plans K or L, in subdivision F 8 or F 9 of this section and list the benefits in the order shown. For purposes of this section, the term "structure, language, and format" means style, arrangement and overall content of a benefit.
E. In addition to the benefit plan designations required in subsection D of this section, an issuer may use other designations to the extent permitted by law.
F. Make-up of 2010 standardized benefit plans:
1. Standardized Medicare supplement benefit Plan A shall include only the basic (core) benefits as defined in 14VAC5-170-75 C.
2. Standardized Medicare supplement benefit Plan B shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible as defined in 14VAC5-170-75 D 1.
3. Standardized Medicare supplement benefit Plan C shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible, skilled nursing facility care, 100% of the Medicare Part B deductible, and medically necessary emergency care in a foreign country as defined in 14VAC5-170-75 D 1, 3, 4 and 6, respectively.
4. Standardized Medicare supplement benefit Plan D shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible, skilled nursing facility care, and medically necessary emergency care in an foreign country as defined in 14VAC5-170-75 D 1, 3 and 6, respectively.
5. Standardized Medicare supplement benefit Plan F shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible, skilled nursing facility care, 100% of the Medicare Part B deductible, 100% of the Medicare Part B excess charges, and medically necessary emergency care in a foreign country as defined in 14VAC5-170-75 D 1, 3, 4, 5 and 6, respectively.
6. Standardized Medicare supplement benefit Plan F With High Deductible shall include only 100% of covered expenses following the payment of the annual deductible as defined in subdivision 6 b of this subsection.
a. The basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible, skilled nursing facility care, 100% of the Medicare Part B deductible, 100% of the Medicare Part B excess charges, and medically necessary emergency care in a foreign country as defined in 14VAC5-170-75 D 1, 3, 4, 5 and 6, respectively.
b. The annual deductible in Plan F With High Deductible shall consist of out-of-pocket expenses, other than premiums, for services covered by Plan F, and shall be in addition to any other specific benefit deductibles. The basis for the deductible shall be $1,500 and shall be adjusted annually from 1999 by the Secretary of the U.S. Department of Health and Human Services to reflect the change in the Consumer Price Index for all urban consumers for the 12-month period ending with August of the preceding year, and rounded to the nearest multiple of $10.
7. Standardized Medicare supplement benefit Plan G shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible, skilled nursing facility care, 100% of the Medicare Part B excess charges, and medically necessary emergency care in a foreign country as defined in 14VAC5-170-75 D 1, 3, 5 and 6, respectively. Effective January 1, 2020, the standardized benefit plans described in 14VAC5-170-87 D 3 (Plan G with High Deductible) may be offered to any individual who was eligible for Medicare prior to January 1, 2020.
8. Standardized Medicare supplement benefit Plan K is mandated by the Medicare Prescription Drug, Improvement and Modernization Act of 2003, and shall include only the following:
a. Part A hospital coinsurance 61st through 90th days: Coverage of 100% of the Part A hospital coinsurance amount for each day used from the 61st through the 90th day in any Medicare benefit period;
b. Part A hospital coinsurance, 91st through 150th days: Coverage of 100% of the Part A hospital coinsurance amount for each Medicare lifetime inpatient reserve day used from the 91st through the 150th day in any Medicare benefit period;
c. Part A hospitalization after 150 days: Upon exhaustion of the Medicare hospital inpatient coverage, including the lifetime reserve days, coverage of 100% of the Medicare Part A eligible expenses for hospitalization paid at the applicable prospective payment system (PPS) rate, or other appropriate Medicare standard of payment, subject to a lifetime maximum benefit of an additional 365 days. The provider shall accept the issuer's payment as payment in full and may not bill the insured for any balance;
d. Medicare Part A deductible: Coverage for 50% of the Medicare Part A inpatient hospital deductible amount per benefit period until the out-of-pocket limitation is met as described in subdivision 8 j of this subsection;
e. Skilled nursing facility care: Coverage for 50% of the coinsurance amount for each day used from the 21st day through the 100th day in a Medicare benefit period for posthospital skilled nursing facility care eligible under Medicare Part A until the out-of-pocket limitation is met as described in subdivision 8 j of this subsection;
f. Hospice care: Coverage for 50% of cost sharing for all Part A Medicare eligible expenses and respite care until the out-of-pocket limitation is met as described in subdivision 8 j of this subsection;
g. Blood: Coverage for 50%, under Medicare Part A or B, of the reasonable cost of the first three pints of blood (or equivalent quantities of packed red blood cells, as defined under federal regulations) unless replaced in accordance with federal regulations until the out-of-pocket limitation is met as described in subdivision 8 j of this subsection;
h. Part B cost sharing: Except for coverage provided in subdivision 8 i of this subsection, coverage for 50% of the cost sharing otherwise applicable under Medicare Part B after the policyholder pays the Part B deductible until the out-of-pocket limitation is met as described in subdivision 8 j of this subsection;
i. Part B preventive services: Coverage of 100% of the cost sharing for Medicare Part B preventive services after the policyholder pays the Part B deductible; and
j. Cost sharing after out-of-pocket limits: Coverage of 100% of all cost sharing under Medicare Parts A and B for the balance of the calendar year after the individual has reached the out-of-pocket limitation on annual expenditures under Medicare Parts A and B of $4,000 in 2006, indexed each year by the appropriate inflation adjustment specified by the Secretary of the U.S. Department of Health and Human Services.
9. Standardized Medicare supplement benefit Plan L is mandated by the Medicare Prescription Drug, Improvement and Modernization Act of 2003, and shall include only the following:
a. The benefits described in subdivisions 8 a, b, c and i of this subsection;
b. The benefit described in subdivisions 8 d, e, f, g and h of this subsection, but substituting 75% for 50%; and
c. The benefit described in subdivision 8 j of this subsection, but substituting $2,000 for $4,000.
10. Standardized Medicare supplement benefit Plan M shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 50% of the Medicare Part A deductible, skilled nursing facility care, and medically necessary emergency care in a foreign country as defined in 14VAC5-170-75 D 2, 3 and 6, respectively.
11. Standardized Medicare supplement benefit Plan N shall include only the basic (core) benefit as defined in 14VAC5-170-75 C, plus 100% of the Medicare Part A deductible, skilled nursing facility care, and medically necessary emergency care in a foreign country as defined in 14VAC5-170-75 D 1, 3 and 6, respectively, with copayments in the following amounts:
a. The lesser of $20 or the Medicare Part B coinsurance or copayment for each covered health care provider office visit (including visits to medical specialists); and
b. The lesser of $50 or the Medicare Part B coinsurance or copayment for each covered emergency room visit; however, this copayment shall be waived if the insured is admitted to any hospital and the emergency visit is subsequently covered as a Medicare Part A expense.
G. New or innovative benefits. An issuer may, with the prior approval of the commission, offer policies or certificates with new or innovative benefits, in addition to the standardized benefits provided in a policy or certificate that otherwise complies with the applicable standards. The new or innovative benefits shall include only benefits that are appropriate to Medicare supplement insurance, are new or innovative, are not otherwise available, and are cost-effective. Approval of new or innovative benefits must not adversely impact the goal of Medicare supplement simplification. New or innovative benefits shall not include an outpatient prescription drug benefit. New or innovative benefits shall not be used to change or reduce benefits, including a change of any cost-sharing provision, in any standardized plan.
14VAC5-170-87. Standard plans for 2020 standardized Medicare supplement policies delivered to individuals newly eligible for Medicare on or after January 1, 2020.
A. This section applies only to individuals who are newly eligible for Medicare on or after January 1, 2020:
1. By reason of attaining age 65 years on or after January 1, 2020; or
2. By reason of entitlement to benefits under part A pursuant to § 226(b) or 226A of the Social Security Act, or who is deemed to be eligible for benefits under § 226(a) of the Social Security Act on or after January 1, 2020.
B. No policy or certificate that provides coverage of the Medicare Part B deductible may be advertised, solicited, delivered, or issued for delivery in the Commonwealth as a Medicare supplement policy or certificate to individuals newly eligible for Medicare on or after January 1, 2020. All such policies must comply with the benefit standards contained in subsection D of this section. Benefit plan standards applicable to Medicare supplement policies and certificates issued to individuals eligible for Medicare before January 1, 2020, remain subject to the requirements of 14VAC5-170-75 and 14VAC5-170-85.
C. Standardized Medicare supplement benefit plans C, F, and F with High Deductible may not be offered to individuals newly eligible for Medicare on or after January 1, 2020. For purposes of this section, the reference to Plans C or F contained in 14VAC5-170-85 B 2 is deemed a reference to Plan D or G, respectively.
D. The standards and requirements of 14VAC5-170-85 shall apply to all Medicare supplement policies or certificates delivered or issued for delivery to individuals newly eligible for Medicare on or after January 1, 2020, with the following exceptions:
1. Standardized Medicare supplement benefit Plan D (previously Plan C) shall provide the benefits contained in 14VAC5-170-85 F 3 but shall not provide coverage for 100% or any portion of the Medicare Part B deductible.
2. Standardized Medicare supplement benefit Plan G (previously Plan F) shall provide the benefits contained in 14VAC5-170-85 F 5 but shall not provide coverage for 100% or any portion of the Medicare Part B deductible.
3. Standardized Medicare supplement benefit Plan G with High Deductible (previously Plan F with High Deductible) shall provide the benefits contained in 14VAC5-170-85 F 6 but shall not provide coverage for 100% or any portion of the Medicare Part B deductible; provided further that the Medicare Part B deductible paid by the beneficiary shall be considered an out-of-pocket expense in meeting the annual high deductible.
E. For purposes of 14VAC5-170-105 E, in the case of any individual newly eligible for Medicare on or after January 1, 2020, any reference to a Medicare supplement policy C or F (including F with High Deductible) shall be deemed to be a reference to Medicare supplement policy D or G (including G with High Deductible), respectively.
14VAC5-170-150. Required disclosure provisions.
A. General rules.
1. Medicare supplement policies and certificates shall include a renewal or continuation provision. The language or specifications of such provision shall be consistent with the type of contract issued. The provision shall be appropriately captioned, shall appear on the first page of the policy, and shall include any reservation by the issuer of the right to change premiums and any automatic renewal premium increases based on the policyholder's age. Medicare supplement policies or certificates which are attained age rated shall include a clear and prominent statement, in at least 14 point type, disclosing that premiums will increase due to changes in age and the frequency under which such changes will occur.
2. Except for riders or endorsements by which the issuer effectuates a request made in writing by the insured, exercises a specifically reserved right under a Medicare supplement policy, or is required to reduce or eliminate benefits to avoid duplication of Medicare benefits, all riders or endorsements added to a Medicare supplement policy after date of issue or at reinstatement or renewal which reduce or eliminate benefits or coverage in the policy shall require a signed acceptance by the insured. After the date of policy or certificate issue, any rider or endorsement which increases benefits or coverage with a concomitant increase in premium during the policy term shall be agreed to in writing signed by the insured, unless the benefits are required by the minimum standards for Medicare supplement policies, or if the increased benefits or coverage is required by law. Where a separate additional premium is charged for benefits provided in connection with riders or endorsements, the premium charge shall be set forth in the policy.
3. Medicare supplement policies or certificates shall not provide for the payment of benefits based on standards described as "usual and customary," "reasonable and customary" or words of similar import.
4. If a Medicare supplement policy or certificate contains any limitations with respect to preexisting conditions, such limitations shall appear as a separate paragraph of the policy and be labeled as "Preexisting Condition Limitations."
5. Medicare supplement policies and certificates shall have a notice prominently printed on the first page of the policy or certificate or attached thereto stating in substance that the policyholder or certificateholder shall have the right to return the policy or certificate within 30 days of its delivery and to have all premiums made for the policy refunded if, after examination of the policy or certificate, the insured person is not satisfied for any reason.
6. Issuers of accident and sickness policies or certificates which provide hospital or medical expense coverage on an expense incurred or indemnity basis to a person or persons eligible for Medicare shall provide to those applicants a Guide to Health Insurance for People with Medicare in the form developed jointly by the National Association of Insurance Commissioners and the Centers for Medicare and Medicaid Services and in a type size no smaller than 12 point type. Delivery of the guide shall be made whether or not such policies or certificates are advertised, solicited or issued as Medicare supplement policies or certificates as defined in this chapter. Except in the case of direct response issuers, delivery of the guide shall be made to the applicant at the time of application and acknowledgement of receipt of the guide shall be obtained by the issuer. Direct response issuers shall deliver the guide to the applicant upon request but not later than at the time the policy is delivered.
For the purposes of this section, "form" means the language, format, type size, type proportional spacing, bold character, and line spacing.
B. Notice requirements.
1. As soon as practicable, but no later than 30 days prior to the annual effective date of any Medicare benefit changes, an issuer shall notify its policyholders and certificateholders of modifications it has made to Medicare supplement insurance policies or certificates in a format acceptable to the State Corporation Commission. The notice shall:
a. Include a description of revisions to the Medicare program and a description of each modification made to the coverage provided under the Medicare supplement policy or certificate; and
b. Inform each policyholder or certificateholder as to when any premium adjustment is to be made due to changes in Medicare.
2. The notice of benefit modifications and any premium adjustments shall be in outline form and in clear and simple terms so as to facilitate comprehension.
3. Such notices shall not contain or be accompanied by any solicitation.
C. Issuers shall comply with any notice requirements of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (42 USC § 1395w-101).
D. Outline of coverage requirements for Medicare Supplement Policies.
1. Issuers shall provide an outline of coverage to all applicants at the time the application is presented to the prospective applicant and, except for direct response policies, shall obtain an acknowledgement of receipt of the outline from the applicant; and
2. If an outline of coverage is provided at the time of application and the Medicare supplement policy or certificate is issued on a basis which would require revision of the outline, a substitute outline of coverage properly describing the policy or certificate shall accompany such policy or certificate when it is delivered and contain the following statement, in no less than 12 point type, immediately above the company name:
"NOTICE: Read this outline of coverage carefully. It is not identical to the outline of coverage provided upon application and the coverage originally applied for has not been issued."
3. The outline of coverage provided to applicants pursuant to this section consists of four parts: a cover page, premium information, disclosure pages, and charts displaying the features of each benefit plan offered by the issuer. The outline of coverage shall be in the language and format prescribed below in no less than 12 point type. All plans shall be shown on the cover page, and the plan(s) plans that are offered by the issuer shall be prominently identified. Premium information for plans that are offered shall be shown on the cover page or immediately following the cover page and shall be prominently displayed. The premium and mode shall be stated for all plans that are offered to the prospective applicant. All possible premiums for the prospective applicant shall be illustrated.
4. The following items shall be included in the outline of coverage in the order prescribed in the following table.
Benefit Chart of Medicare Supplement Plans Sold with Effective Dates on or after June 1, 2010
This chart shows the benefits included in each of the standard Medicare supplement plans. Every company must make Plan A available.
Some plans may not be available in your state.
Plans E, H, I and J are no longer available for sale after June 1, 2010. [This sentence shall not appear after June 1, 2011.]
Plans C, F, and high deductible F are no longer available for sale to those who are newly eligible, as defined in 14VAC5-170-87, on or after January 1, 2020.
Note that the numerical figures in the following charts, including out-of-pocket limits and deductible amounts, are current as of January 1, 2018, and are subject to change.
Basic benefits:
Hospitalization – Part A coinsurance plus coverage for 365 additional days after Medicare benefits end.
Medical expenses – Part B coinsurance (generally 20% of Medicare-approved expenses) or copayments for hospital outpatient services. Plans K, L and N require insureds to pay a portion of Part B coinsurance or copayments.
Blood – First three pints of blood each year.
Hospice – Part A coinsurance.
A | B | C | D | F | F* | G | K | L | M | N |
Basic,
including 100% Part B coinsurance | Basic,
including 100% Part B coinsurance | Basic,
including 100% Part B coinsurance | Basic,
including 100% Part B coinsurance | Basic,
including 100% Part B coinsurance* | Basic,
including 100% Part B coinsurance | Hospitalization and preventive care paid at 100%; other basic benefits paid at 50% | Hospitalization and preventive care paid at 100%; other basic benefits paid at 75% | Basic,
including 100% Part B coinsurance | Basic,
including 100% Part B coinsurance, except up to $20 copayment for office visit, and up to $50 copayment for ER |
| | Skilled nursing facility coinsurance | Skilled nursing facility coinsurance | Skilled nursing facility coinsurance | Skilled nursing facility coinsurance | 50% skilled nursing facility coinsurance | 75% skilled nursing facility coinsurance | Skilled nursing facility coinsurance | Skilled nursing facility coinsurance |
| Part A deductible | Part A deductible | Part A deductible | Part A deductible | Part A deductible | 50% Part A deductible | 75% Part A deductible | 50% Part A deductible | Part A deductible |
| | Part B deductible | | Part B deductible | | | | | |
| | | | Part B excess (100%) | Part B excess (100%) | | | | |
| | Foreign travel emergency | Foreign travel emergency | Foreign travel emergency | Foreign travel emergency | | | Foreign travel emergency | Foreign travel emergency |
| | | | | | Out-of-pocket limit $4,620 $4,940; paid at 100% after limit reached | Out-of-pocket limit $2,310 $2,470; paid at 100% after limit reached | | |
*Plan F also has an option called a high deductible Plan F. This high deductible plan pays the same benefits as Plan F after one has paid a calendar year $2,000 $2,180 deductible. Benefits from high deductible Plan F will not begin until out-of-pocket expenses exceed $2,000 $2,180. Out-of-pocket expenses for this deductible are expenses that would ordinarily be paid by the policy. These expenses include the Medicare deductibles for Part A and Part B, but do not include the plan's separate foreign travel emergency deductible.
PREMIUM INFORMATION
Boldface Type
We [insert issuer's name] can only raise your premium if we raise the premium for all policies like yours in this Commonwealth. [If the premium is based on attained age of the insured, include the following information:
1. When premiums will change;
2. The current premium for all ages;
3. A statement that premiums for other Medicare Supplement policies that are issue age or community rated do not increase due to changes in your age; and
4. A statement that while the cost of this policy at the covered individual's present age may be lower than the cost of a Medicare supplement policy that is based on issue age or community rated, it is important to compare the potential cost of these policies over the life of the policy.]
DISCLOSURES
Boldface Type
Use this outline to compare benefits and premiums among policies.
This outline shows benefits and premiums of policies sold for effective dates on or after June 1, 2010. Policies sold for effective dates prior to June 1, 2010, have different benefits and premiums. Plans E, H, I and J are no longer available for sale after June 1, 2010. [This paragraph shall not appear after June 1, 2011.]
READ YOUR POLICY VERY CAREFULLY
Boldface Type
This is only an outline describing your policy's most important features. The policy is your insurance contract. You must read the policy itself to understand all of the rights and duties of both you and your insurance company.
RIGHT TO RETURN POLICY
Boldface Type
If you find that you are not satisfied with your policy, you may return it to [insert issuer's address]. If you send the policy back to us within 30 days after you receive it, we will treat the policy as if it had never been issued and return all of your payments.
POLICY REPLACEMENT
Boldface Type
If you are replacing another health insurance policy, do NOT cancel it until you have actually received your new policy and are sure you want to keep it.
NOTICE
Boldface Type
This policy may not fully cover all of your medical costs.
[for agents:]
Neither [insert company's name] nor its agents are connected with Medicare.
[for direct response:]
[insert company's name] is not connected with Medicare.
This outline of coverage does not give all the details of Medicare coverage. Contact your local Social Security Office or consult "Medicare & You" for more details.
COMPLETE ANSWERS ARE VERY IMPORTANT
Boldface Type
When you fill out the application for the new policy, be sure to answer truthfully and completely all questions about your medical and health history. The company may cancel your policy and refuse to pay any claims if you leave out or falsify important medical information. [If the policy or certificate is guaranteed issue, this paragraph need not appear.]
Review the application carefully before you sign it. Be certain that all information has been properly recorded.
[Include for each plan prominently identified in the cover page, a chart showing the services, Medicare payments, plan payments and insured payments for each plan, using the same language, in the same order, using uniform layout and format as shown in the charts below. No more than four plans may be shown on one chart. For purposes of illustration, charts for each plan are included in this regulation. An issuer may use additional benefit plan designations on these charts pursuant to 14VAC5-170-85.]
[Include an explanation of any innovative benefits on the cover page and in the chart, in a manner approved by the State Corporation Commission.]
Benefit Chart of Medicare Supplement Plans Sold on or after January 1, 2020
This chart shows the benefits included in each of the standard Medicare supplement plans. Some plans may not be available. Only applicants first eligible for Medicare before 2020 may purchase Plans C, F, and high deductible F.
Note: A ✔means 100% of the benefit is paid.
Benefits | Plans Available to All Applicants | | Medicare first eligible before 2020 only |
A | B | D | G1 | K | L | M | N | | C | F1 |
Medicare Part A coinsurance and hospital coverage (up to an additional 365 days after Medicare benefits are used up) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | | ✔ | ✔ |
Medicare Part B coinsurance or copayment | ✔ | ✔ | ✔ | ✔ | 50% | 75% | ✔ | ✔copays apply3 | | ✔ | ✔ |
Blood (first three pints) | ✔ | ✔ | ✔ | ✔ | 50% | 75% | ✔ | ✔ | | ✔ | ✔ |
Part A hospice care coinsurance or copayment | ✔ | ✔ | ✔ | ✔ | 50% | 75% | ✔ | ✔ | | ✔ | ✔ |
Skilled nursing facility coinsurance | | | ✔ | ✔ | 50% | 75% | ✔ | ✔ | | ✔ | ✔ |
Medicare Part A deductible | | ✔ | ✔ | ✔ | 50% | 75% | 50% | ✔ | | ✔ | ✔ |
Medicare Part B deductible | | | | | | | | | | ✔ | ✔ |
Medicare Part B excess charges | | | | ✔ | | | | | | | ✔ |
Foreign travel emergency (up to plan limits) | | | ✔ | ✔ | | | ✔ | ✔ | | ✔ | ✔ |
Out-of-pocket limit in 20162 | | | | | $4,9602 | $2,4802 | | | | | |
1 Plans F and G also have a high deductible option that require first paying a plan deductible of $2,180 before the plan begins to pay. Once the plan deductible is met, the plan pays 100% of covered services for the rest of the calendar year. High deductible Plan G does not cover the Medicare Part B deductible. However, high deductible Plans F and G count your payment of the Medicare Part B deductible toward meeting the plan deductible. High deductible Plan G is the same as high deductible Plan F except that where the annual out-of-pocket expenses are met with Medicare Part A expenses only, any subsequent Medicare Part B deductible expense incurred by the beneficiary after the required annual out-of-pocket expenses is met may not be paid for by the high deductible Plan G.
2 Plans K and L pay 100% of covered services for the rest of the calendar year once you meet the out-of-pocket yearly limit.
3 Plan N pays 100% of the Part B coinsurance, except for a copayment of up to $20 for some office visits and up to a $50 copayment for emergency room visits that do not result in an inpatient admission.
PLAN A
MEDICARE (PART A) - HOSPITAL SERVICES - PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $0 | $1,068 $1,260 (Part A Deductible)
|
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare Eligible Expenses | $0** |
| Beyond the Additional 365 days | $0 | $0 | All Costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | $0 | Up to $133.50 $157.50 a day |
101st day and after | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN A
MEDICARE (PART B) - MEDICAL SERVICES - PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-Approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 20% | $0 |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | All Costs | $0 |
Next $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
PLAN B
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $1,068 $1,260 (Part A Deductible)
| $0 |
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare Eligible Expenses | $0** |
| Beyond the Additional 365 days | $0 | $0 | All Costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | $0 | Up to $133.50 $157.50 a day |
101st day and after | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN B
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-Approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
| SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
| MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
| Remainder of Medicare-Approved Amounts | Generally 80% | Generally 20% | $0 |
| PART B EXCESS CHARGES | | | |
| (Above Medicare-Approved Amounts) | $0 | $0 | All Costs |
| BLOOD | | | |
| First 3 pints | $0 | All Costs | $0 |
| Next $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
| CLINICAL LABORATORY SERVICES - | | | |
| TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
PLAN C
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $,1068 $1,260 (Part A Deductible)
| $0 |
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare eligible expenses | $0** |
| Beyond the additional 365 days | $0 | $0 | All Costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $133.50 $157.50 a day | $0 |
101st day and after | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN C
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-Approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts* | $0 | $135 $147 (Part B Deductible)
| $0 |
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 20% | $0 |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | All Costs | $0 |
Next $135 $147 of Medicare-Approved Amounts* | $0 | $135 $147 (Part B Deductible)
| $0 |
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $135 $147 (Part B Deductible)
| $0 |
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
OTHER BENEFITS—NOT COVERED BY MEDICARE
FOREIGN TRAVEL
NOT COVERED BY MEDICARE | | | |
Medically necessary emergency care services beginning during the first 60 days of each trip outside the USA | | | |
| First $250 each calendar year | $0 | $0 | $250 |
| Remainder of Charges | $0 | 80% to a lifetime maximum benefit of $50,000 | 20% and amounts over the $50,000 lifetime maximum |
PLAN D
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $1,068 $1,260 (Part A Deductible)
| $0 |
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare Eligible Expenses | $0** |
| Beyond the Additional 365 days | $0 | $0 | All Costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $133.50 $157.50 a day | $0 |
101st day and after | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN D
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-Approved amounts for covered services (which are noted with an asterisk), your Part B Deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 20% | $0 |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | All Costs | $0 |
Next $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B Deductible)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
OTHER BENEFITS—NOT COVERED BY MEDICARE
FOREIGN TRAVEL
NOT COVERED BY MEDICARE | | | |
Medically necessary emergency care services beginning during the first 60 days of each trip outside the USA | | | |
| First $250 each calendar year | $0 | $0 | $250 |
| Remainder of Charges | $0 | 80% to a lifetime maximum benefit of $50,000 | 20% and amounts over the $50,000 lifetime maximum |
PLAN F or HIGH DEDUCTIBLE PLAN F
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
**This high deductible plan pays the same benefits as Plan F after one has you have paid a calendar year $2,000 $2,180 deductible. Benefits from the high deductible Plan F will not begin until out-of-pocket expenses are $2,000 $2,180. Out-of-pocket expenses for this deductible are expenses that would ordinarily be paid by the policy. This includes the Medicare deductibles for Part A and Part B, but does not include the plan's separate foreign travel emergency deductible.
SERVICES | MEDICARE PAYS | AFTER YOU PAY $2,000 $2,180 DEDUCTIBLE,** PLAN PAYS | IN ADDITION TO $2,000 $2,180 DEDUCTIBLE,** YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $1,068 $1,260 (Part A Deductible)
| $0 |
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare Eligible Expenses | $0** |
| Beyond the Additional 365 days | $0 | $0 | All Costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $133.50 $157.50 a day | $0 |
101st day and after | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN F or HIGH DEDUCTIBLE PLAN F
MEDICARE (PART B) - MEDICAL SERVICES - PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-Approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
**This high deductible plan pays the same benefits as Plan F after one has you have paid a calendar year $2,000 $2,180 deductible. Benefits from the high deductible plan F will not begin until out-of-pocket expenses are $2,000 $2,180. Out-of-pocket expenses for this deductible are expenses that would ordinarily be paid by the policy. This includes the Medicare deductibles for Part A and Part B, but does not include the plan's separate foreign travel emergency deductible.
SERVICES | MEDICARE PAYS | AFTER YOU PAY $2,000 $2,180 DEDUCTIBLE,** PLAN PAYS | IN ADDITION ADDITION TO $2,000 $2,180 DEDUCTIBLE,** YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved amounts* | $0 | $135 $147 (Part B Deductible)
| $0 |
Remainder of Medicare-Approved amounts | Generally 80% | Generally 20% | $0 |
PART B EXCESS CHARGES | | | |
(Above Medicare Approved Amounts) | $0 | 100% | $0 |
BLOOD | | | |
First 3 pints | $0 | All Costs | $0 |
Next $135 $147 of Medicare-Approved Amounts* | $0 | $135 $147 (Part B Deductible)
| $0 |
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | AFTER YOU PAY $2,000 $2,180 DEDUCTIBLE,** PLAN PAYS | IN ADDITON ADDITION TO $2,000 $2,180 DEDUCTIBLE,** YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $135 $147 (Part B Deductible)
| $0 |
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
OTHER BENEFITS - NOT COVERED BY MEDICARE
FOREIGN TRAVEL
NOT COVERED BY MEDICARE | | | |
Medically necessary emergency care services beginning during the first 60 days of each trip outside the USA | | | |
| First $250 each calendar year | $0 | $0 | $250 |
| Remainder of charges | $0 | 80% to a lifetime maximum benefit of $50,000 | 20% and amounts over the $50,000 lifetime maximum |
PLAN G OR HIGH DEDUCTIBLE PLAN G
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | AFTER YOU PAY $2,180 DEDUCTIBLE, PLAN PAYS | IN ADDITION TO $2,180 DEDUCTIBLE, YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,288 | $1,068 $1,288 (Part A Deductible)
| $0 |
61st thru 90th day | All but $267 $322 a day | $267 $322 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $644 a day | $534 $644 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare Eligible Expenses | $0** |
| Beyond the Additional 365 days | $0 | $0 | All costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $161 a day | Up to $133.50 $161 a day | $0 |
101st day and after | $0 | $0 | All Costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN G OR HIGH DEDUCTIBLE PLAN G
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
*Once you have been billed $135 $166 of Medicare-Approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $166 of Medicare-Approved Amounts* | $0 | $0 | $135 (Part B Deductible) $166 (Unless Part B Deductible has been met)
|
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 20% | $0 |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | 100% | $0 |
BLOOD | | | |
First 3 pints | $0 | All costs | $0 |
Next $135 $166 of Medicare-Approved Amounts* | $0 | $0 | $135 (Part B Deductible) $166 (Unless Part B Deductible has been met)
|
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $166 of Medicare-Approved Amounts* | $0 | $0 | $135 (Part B deductible) $166 (Unless Part B Deductible has been met)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
OTHER BENEFITS—NOT COVERED BY MEDICARE
FOREIGN TRAVEL
NOT COVERED BY MEDICARE | | | |
Medically necessary emergency care services beginning during the first 60 days of each trip outside the USA | | | |
| First $250 each calendar year | $0 | $0 | $250 |
| Remainder of Charges | $0 | 80% to a lifetime maximum benefit of $50,000 | 20% and amounts over the $50,000 lifetime maximum |
PLAN K
*You will pay half the cost-sharing of some covered services until you reach the annual out-of-pocket limit of $4,620 $4,940 each calendar year. The amounts that count toward your annual limit are noted with diamonds (♦) in the chart below. Once you reach the annual limit, the plan pays 100% of your Medicare copayment and coinsurance for the rest of the calendar year. However, this limit does NOT include charges from your provider that exceed Medicare-approved amounts (these are called "Excess Charges") and you will be responsible for paying this difference in the amount charged by your provider and the amount paid by Medicare for the item or service.
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
**A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY* |
HOSPITALIZATION** Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $534 $630 (50% of Part A deductible)
| $534 $630 (50% of Part A deductible)♦
|
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare eligible expenses | $0*** |
| Beyond the additional 365 days | $0 | $0 | All costs |
SKILLED NURSING FACILITYCARE** You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $66.75 $78.75 a day (50% of Part A coinsurance) | Up to $66.75 $78.75 a day (50% of Part A coinsurance)♦ |
101st day and after | $0 | $0 | All costs |
BLOOD | | | |
First 3 pints | $0 | 50% | 50%♦ |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | 50% of copayment/coinsurance | 50% of Medicare copayment/coinsurance ♦ |
| | | | | |
***NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever the amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charges and the amount Medicare would have paid.
PLAN K
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
****Once you have been billed $135 $147 of Medicare-approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY* |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts**** | $0 | $0 | $135 $147 (Part B deductible)****♦
|
Preventive Benefits for Medicare covered services | Generally 75% 80% or more of Medicare-approved amounts | Remainder of Medicare-approved amounts | All costs above Medicare-approved amounts |
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 10% | Generally 10%♦ |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All costs (and they do not count toward annual out-of-pocket limit of $4620)* $4,940)* |
BLOOD | | | |
First 3 pints | $0 | 50% | 50%♦ |
Next $135 $147 of Medicare Approved Amounts**** | $0 | $0 | $135 $147 (Part B deductible)****♦
|
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 10% | Generally 10%♦ |
CLINICAL LABORATORY SERVICES - | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
*This plan limits your annual out-of-pocket payments for Medicare-approved amounts to $4,620 $4,940 per year. However, this limit does NOT include charges from your provider that exceed Medicare-approved amounts (these are called "Excess Charges") and you will be responsible for paying this difference in the amount charged by your provider and the amount paid by Medicare for the item or service.
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY* |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts***** | $0 | $0 | $135 $147 (Part B deductible)♦
|
| Remainder of Medicare-Approved Amounts | 80% | 10% | 10%♦ |
*****Medicare benefits are subject to change. Please consult the latest Guide to Health Insurance for People with Medicare.
PLAN L
*You will pay one-fourth of the cost-sharing of some covered services until you reach the annual out-of-pocket limit of $2,310 $2,470 each calendar year. The amounts that count toward your annual limit are noted with diamonds (♦) in the chart below. Once you reach the annual limit, the plan pays 100% of your Medicare copayment and coinsurance for the rest of the calendar year. However, this limit does NOT include charges from your provider that exceed Medicare-approved amounts (these are called "Excess Charges") and you will be responsible for paying this difference in the amount charged by your provider and the amount paid by Medicare for the item or service.
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
**A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY* |
HOSPITALIZATION** Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $808.50 $945 (75% of Part A deductible)
| $267 $315 (25% of Part A deductible)♦
|
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare eligible expenses | $0*** |
| Beyond the additional 365 days | $0 | $0 | All costs |
SKILLED NURSING FACILITY CARE** You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $100.13 $118.13 a day (75% of Part A coinsurance) | Up to $33.38 $39.38 a day (25% of Part A coinsurance)♦ |
101st day and after | $0 | $0 | All costs |
BLOOD | | | |
First 3 pints | $0 | 75% | 25%♦ |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | 75% of copayment/coinsurance | 25% of copayment/coinsurance ♦ |
| | | | | |
***NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charge and the amount Medicare would have paid.
PLAN L
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
****Once you have been billed $135 $147 of Medicare-approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY* |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts**** | $0 | $0 | $135 $147 (Part B deductible)****♦
|
Preventive Benefits for Medicare covered services | Generally 75% 80% or more of Medicare-approved amounts | Remainder of Medicare-approved amounts | All costs above Medicare-approved amounts |
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 15% | Generally 5%♦ |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All costs (and they do not count toward annual out-of-pocket limit of $2,310)* $2,470)* |
BLOOD | | | |
First 3 pints | $0 | 75% | 25%♦ |
Next $135 $147 of Medicare Approved Amounts**** | $0 | $0 | $135 $147 (Part B deductible)♦
|
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 15% | Generally 5%♦ |
CLINICAL LABORATORY SERVICES - | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
*This plan limits your annual out-of-pocket payments for Medicare-approved amounts to $2,310 $2,470 per year. However, this limit does NOT include charges from your provider that exceed Medicare-approved amounts (these are called "Excess Charges") and you will be responsible for paying this difference in the amount charged by your provider and the amount paid by Medicare for the item or service.
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY* |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts***** | $0 | $0 | $135 $147 (Part B deductible)♦
|
| Remainder of Medicare-Approved Amounts | 80% | 15% | 5%♦ |
*****Medicare benefits are subject to change. Please consult the latest Guide to Health Insurance for People with Medicare.
PLAN M
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $534 $630 (50% of Part A deductible)
| $534 $630 (50% of Part A deductible)
|
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare eligible expenses | $0** |
| Beyond the additional 365 days | $0 | $0 | All costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $133.50 $157.50 a day | $0 |
101st day and after | $0 | $0 | All costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charge and the amount Medicare would have paid.
PLAN M
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
Remainder of Medicare-Approved Amounts | Generally 80% | Generally 20% | $0 |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All costs |
BLOOD | | | |
First 3 pints | $0 | All costs | $0 |
Next $135 $147 of Medicare Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
OTHER BENEFITS—NOT COVERED BY MEDICARE
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
FOREIGN TRAVEL
NOT COVERED BY MEDICARE | | | |
Medically necessary emergency care services beginning during the first 60 days of each trip outside the USA | | | |
| First $250 each calendar year | $0 | $0 | $250 |
| Remainder of Charges | $0 | 80% to a lifetime maximum benefit of $50,000 | 20% and amounts over the $50,000 lifetime maximum |
PLAN N
MEDICARE (PART A)—HOSPITAL SERVICES—PER BENEFIT PERIOD
*A benefit period begins on the first day you receive service as an inpatient in a hospital and ends after you have been out of the hospital and have not received skilled care in any other facility for 60 days in a row.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOSPITALIZATION* Semiprivate room and board, general nursing and miscellaneous services and supplies | | | |
First 60 days | All but $1,068 $1,260 | $1,068 $1,260 (Part A deductible)
| $0 |
61st thru 90th day | All but $267 $315 a day | $267 $315 a day
| $0 |
91st day and after: | | | |
| While using 60 lifetime reserve days | All but $534 $630 a day | $534 $630 a day
| $0 |
| Once lifetime reserve days are used: | | | |
| Additional 365 days | $0 | 100% of Medicare eligible expenses | $0** |
| Beyond the additional 365 days | $0 | $0 | All costs |
SKILLED NURSING FACILITY CARE* You must meet Medicare's requirements, including having been in a hospital for at least 3 days and entered a Medicare-approved facility within 30 days after leaving the hospital | | | |
First 20 days | All approved amounts | $0 | $0 |
21st thru 100th day | All but $133.50 $157.50 a day | Up to $133.50 $157.50 a day | $0 |
101st day and after | $0 | $0 | All costs |
BLOOD | | | |
First 3 pints | $0 | 3 pints | $0 |
Additional amounts | 100% | $0 | $0 |
HOSPICE CARE | | | |
You must meet Medicare's requirements, including a doctor's certification of terminal illness. | All but very limited copayment/coinsurance for outpatient drugs and inpatient respite care | Medicare copayment/coinsurance | $0 |
| | | | | |
**NOTICE: When your Medicare Part A hospital benefits are exhausted, the insurer stands in the place of Medicare and will pay whatever amount Medicare would have paid for up to an additional 365 days as provided in the policy's "core benefits." During this time the hospital is prohibited from billing you for the balance based on any difference between its billed charge and the amount Medicare would have paid.
PLAN N
MEDICARE (PART B)—MEDICAL SERVICES—PER CALENDAR YEAR
*Once you have been billed $135 $147 of Medicare-approved amounts for covered services (which are noted with an asterisk), your Part B deductible will have been met for the calendar year.
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
MEDICAL EXPENSES - IN OR OUT OF THE HOSPITAL AND OUTPATIENT HOSPITAL TREATMENT, such as physician's services, inpatient and outpatient medical and surgical services and supplies, physical and speech therapy, diagnostic tests, durable medical equipment | | | |
First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
Remainder of Medicare-Approved Amounts | Generally 80% | Balance, other than up to $20 per office visit and up to $50 per emergency room visit. The copayment of up to $50 is waived if the insured is admitted to any hospital and the emergency visit is covered as a Medicare Part A expense. | Up to$20 per office visit and up to $50 per emergency visit. The copayment of up to $50 is waived if the insured is admitted to any hospital and the emergency room visit is covered as a Medicare Part A expense. |
PART B EXCESS CHARGES | | | |
(Above Medicare-Approved Amounts) | $0 | $0 | All costs |
BLOOD | | | |
First 3 pints | $0 | All costs | $0 |
Next $135 $147 of Medicare Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
CLINICAL LABORATORY SERVICES - | | | |
TESTS FOR DIAGNOSTIC SERVICES | 100% | $0 | $0 |
PARTS A & B
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
HOME HEALTH CARE
MEDICARE-APPROVED SERVICES | | | |
Medically necessary skilled care services and medical supplies | 100% | $0 | $0 |
Durable medical equipment | | | |
| First $135 $147 of Medicare-Approved Amounts* | $0 | $0 | $135 $147 (Part B deductible)
|
| Remainder of Medicare-Approved Amounts | 80% | 20% | $0 |
OTHER BENEFITS—NOT COVERED BY MEDICARE
SERVICES | MEDICARE PAYS | PLAN PAYS | YOU PAY |
FOREIGN TRAVEL
NOT COVERED BY MEDICARE | | | |
Medically necessary emergency care services beginning during the first 60 days of each trip outside the USA | | | |
| First $250 each calendar year | $0 | $0 | $250 |
| Remainder of Charges | $0 | 80% to a lifetime maximum benefit of $50,000 | 20% and amounts over the $50,000 lifetime maximum |
E. Notice regarding policies or certificates which that are not Medicare supplement policies.
1. Any accident and sickness insurance policy or certificate issued for delivery in this Commonwealth to persons eligible for Medicare, other than a Medicare supplement policy, a policy issued pursuant to a contract under § 1876 of the federal Social Security Act (42 USC § 1395 et seq.), a disability income policy, or other policy identified in 14VAC5-170-20 B, shall notify insureds under the policy that the policy is not a Medicare supplement policy or certificate. The notice shall either be printed or attached to the first page of the outline of coverage delivered to insureds under the policy, or if no outline of coverage is delivered, to the first page of the policy, or certificate delivered to insureds. The notice shall be in no less than 12 point type and shall contain the following language:
"THIS [POLICY OR CERTIFICATE] IS NOT A MEDICARE SUPPLEMENT [POLICY OR CONTRACT]. If you are eligible for Medicare, review the Guide to Health Insurance for People with Medicare available from the company."
2. Applications provided to persons eligible for Medicare for the health insurance policies or certificates described in subdivision 1 of this subsection shall disclose, using the applicable statement in Appendix C, the extent to which the policy duplicates Medicare. The disclosure statement shall be provided as a part of, or together with, the application for the policy or certificate.
F. Notice requirements for attained age rated Medicare supplement policies or certificates. Issuers of Medicare supplement policies or certificates which that use attained age rating shall provide a notice to all prospective applicants at the time the application is presented, and except for direct response policies or certificates, shall obtain an acknowledgement of receipt of the notice from the applicant. The notice shall be in no less than 12 point type and shall contain the information included in Appendix D. The notice shall be provided as part of, or together with, the application for the policy or certificate.
VA.R. Doc. No. R17-5121; Filed August 21, 2017, 2:56 p.m.
TITLE 18. PROFESSIONAL AND OCCUPATIONAL LICENSING
BOARD OF MEDICINE
Final Regulation
REGISTRAR'S NOTICE: The
Board of Medicine is claiming an exemption from Article 2 of the Administrative
Process Act in accordance with § 2.2-4006 A 4 a of the Code of Virginia,
which excludes regulations that are necessary to conform to changes in Virginia
statutory law where no agency discretion is involved. The Board of Medicine
will receive, consider, and respond to petitions by any interested person at
any time with respect to reconsideration or revision.
Title of Regulation: 18VAC85-20. Regulations
Governing the Practice of Medicine, Osteopathic Medicine, Podiatry, and
Chiropractic (amending 18VAC85-20-122).
Statutory Authority: § 54.1-2400 of the Code of
Virginia.
Effective Date: October 18, 2017.
Agency Contact: William L. Harp, M.D., Executive
Director, Board of Medicine, 9960 Mayland Drive, Suite 300, Richmond, VA
23233-1463, telephone (804) 367-4621, FAX (804) 527-4429, or email william.harp@dhp.virginia.gov.
Summary:
Pursuant to Chapters 59 and 117 of the 2017 Acts of
Assembly, the amendments eliminate the requirement of two years of training for
foreign graduates and require one year for all graduates.
18VAC85-20-122. Educational requirements: Graduates and former
students of institutions not approved by an accrediting agency recognized by
the board.
A. A graduate of an institution not approved by an
accrediting agency recognized by the board shall present documentary evidence
that he:
1. Was enrolled and physically in attendance at the
institution's principal site for a minimum of two consecutive years and
fulfilled at least half of the degree requirements while enrolled two
consecutive academic years at the institution's principal site.
2. Has fulfilled the applicable requirements of § 54.1-2930
of the Code of Virginia.
3. Has obtained a certificate from the Educational Council of
Foreign Medical Graduates (ECFMG), or its equivalent. Proof of licensure by the
board of another state or territory of the United States or a province of
Canada may be accepted in lieu of ECFMG certification.
4. Has had supervised clinical training as a part of his
curriculum in an approved hospital, institution or school of medicine offering
an approved residency program in the specialty area for the clinical training
received or in a program acceptable to the board and deemed a substantially
equivalent experience, if such training was received in the United States.
5. Has completed two years one year of
satisfactory postgraduate training as an intern, resident, or clinical fellow.
The two years one year shall include at least 12 months in one
program or institution approved by an accrediting agency recognized by the
board for internship or residency training or in a clinical fellowship
acceptable to the board in the same or a related field.
a. The board may substitute other postgraduate training or
study for one year of the two-year requirement when such training or study has
occurred in the United States or Canada and is:
(1) An approved fellowship program; or
(2) A position teaching medical students, interns, or
residents in a medical school program approved by an accrediting agency
recognized by the board for internship and residency training.
b. The board may substitute continuous full-time
practice of five years or more with a limited professorial license in Virginia
and one year of postgraduate training in a foreign country in lieu of two
years one year of postgraduate training.
6. Has received a degree from the institution.
B. A former student who has completed all degree requirements
except social services and postgraduate internship at a school not approved by
an accrediting agency recognized by the board shall be considered for licensure
provided that he:
1. Has fulfilled the requirements of subdivisions A 1 through
5 of this section;
2. Has qualified for and completed an appropriate supervised
clinical training program as established by the American Medical Association;
and
3. Presents a document issued by the school certifying that he
has met all the formal requirements of the institution for a degree except
social services and postgraduate internship.
VA.R. Doc. No. R18-5232; Filed August 21, 2017, 10:42 a.m.
TITLE 18. PROFESSIONAL AND OCCUPATIONAL LICENSING
BOARD OF MEDICINE
Emergency Regulation
Title of Regulation: 18VAC85-21. Regulations
Governing Prescribing of Opioids and Buprenorphine (amending 18VAC85-21-30, 18VAC85-21-40,
18VAC85-21-60, 18VAC85-21-70, 18VAC85-21-80, 18VAC85-21-130, 18VAC85-21-140,
18VAC85-21-150, 18VAC85-21-160).
Statutory Authority: §§ 54.1-2400 and 54.1-2928.2
of the Code of Virginia.
Effective Dates: August 24, 2017, through September 14,
2018.
Agency Contact: William L. Harp, M.D., Executive
Director, Board of Medicine, 9960 Mayland Drive, Suite 300, Richmond, VA 23233,
telephone (804) 367-4558, FAX (804) 527-4429, or email
william.harp@dhp.virginia.gov.
Preamble:
Section 2.2-4011 of the Code of Virginia authorizes an
agency to adopt emergency regulations upon consultation with the Attorney
General, and the necessity for the action is at the sole discretion of the
Governor. Emergency regulations for practitioners prescribing medication
containing opioids and products containing buprenorphine were published in 33:16 VA.R. 1928-1932 April 3, 2017,
and became effective March 15, 2017.
The Board of Medicine adopted amendments to the emergency
regulation that became effective March 15, 2017. The amendments (i) prohibit
prescribing buprenorphine mono-product in tablet form for chronic pain; (ii)
clarify the limitations on prescribing buprenorphine mono-product for patients
with a demonstrated intolerance to naloxone; (iii) permit prescribing
buprenorphine mono-product for a pregnant woman if medically indicated; and
(iv) replace the term "abuse" with "misuse."
The purpose of the amendments is to provide patients who
have a demonstrated intolerance to naloxone with access to buprenorphine in the
treatment of substance misuse and provide prescribers with definitive rules to
follow so that prescribers are more assured of their ability to treat pain in
an appropriate manner to avoid under-prescribing or over-prescribing.
18VAC85-21-30. Evaluation of the acute pain patient.
A. Nonpharmacologic and non-opioid treatment for pain
shall be given consideration prior to treatment with opioids. If an opioid is
considered necessary for the treatment of acute pain, the practitioner shall
give a short-acting opioid in the lowest effective dose for the fewest possible
days.
B. Prior to initiating treatment with a controlled
substance containing an opioid for a complaint of acute pain, the prescriber
shall perform a history and physical examination appropriate to the complaint,
query the Prescription Monitoring Program as set forth in § 54.1-2522.1 of the
Code of Virginia, and conduct an assessment of the patient's history and risk
of substance [ abuse misuse ].
18VAC85-21-40. Treatment of acute pain with opioids.
A. Initiation of opioid treatment for patients with acute
pain shall be with short-acting opioids.
1. A prescriber providing treatment for acute pain shall
not prescribe a controlled substance containing an opioid in a quantity that
exceeds a seven-day supply as determined by the manufacturer's directions for
use, unless extenuating circumstances are clearly documented in the
medical record. This shall also apply to prescriptions of a controlled
substance containing an opioid upon discharge from an emergency department.
2. An opioid prescribed as part of treatment for a surgical
procedure shall be for no more than 14 consecutive days in accordance with
manufacturer's direction and within the immediate perioperative period, unless
extenuating circumstances are clearly documented in the medical record.
B. Initiation of opioid treatment for all patients shall
include the following:
1. The practitioner shall carefully consider and document
in the medical record the reasons to exceed 50 MME/day.
2. Prior to exceeding 120 MME/day, the practitioner shall
document in the medical record the reasonable justification for such
doses or refer to or consult with a pain management specialist.
3. Naloxone shall be prescribed for any patient when risk
factors of prior overdose, substance [ abuse misuse ],
doses in excess of 120 MME/day, or concomitant benzodiazepine is present.
C. Due to a higher risk of fatal overdose when opioids are
prescribed with benzodiazepines, sedative hypnotics, carisoprodol, and
tramadol, the prescriber shall only co-prescribe these substances when there
are extenuating circumstances and shall document in the medical record a
tapering plan to achieve the lowest possible effective doses if these
medications are prescribed.
D. Buprenorphine is not indicated for acute pain in the
outpatient setting, except when a prescriber who has obtained a SAMHSA waiver
is treating pain in a patient whose primary diagnosis is the disease of
addiction.
18VAC85-21-60. Evaluation of the chronic pain patient.
A. Prior to initiating management of chronic pain with a
controlled substance containing an opioid, a medical history and physical
examination, to include a mental status examination, shall be performed and
documented in the medical record, including:
1. The nature and intensity of the pain;
2. Current and past treatments for pain;
3. Underlying or coexisting diseases or conditions;
4. The effect of the pain on physical and psychological
function, quality of life, and activities of daily living;
5. Psychiatric, addiction, and substance [ abuse
misuse ] history of the patient and any family history of addiction
or substance [ abuse misuse ];
6. A urine drug screen or serum medication level;
7. A query of the Prescription Monitoring Program as set
forth in § 54.1-2522.1 of the Code of Virginia;
8. An assessment of the patient's history and risk of
substance [ abuse misuse ]; and
9. A request for prior applicable records.
B. Prior to initiating opioid treatment for chronic pain,
the practitioner shall discuss with the patient the known risks and benefits of
opioid therapy and the responsibilities of the patient during treatment to
include securely storing the drug and properly disposing of any unwanted or
unused drugs. The practitioner shall also discuss with the patient an exit
strategy for the discontinuation of opioids in the event they are not
effective.
18VAC85-21-70. Treatment of chronic pain with opioids.
A. Nonpharmacologic and non-opioid treatment for pain
shall be given consideration prior to treatment with opioids.
B. In initiating and treating with an opioid, the
practitioner shall:
1. Carefully consider and document in the medical record
the reasons to exceed 50 MME/day;
2. Prior to exceeding 120 MME/day, the practitioner shall
document in the medical record the reasonable justification for such doses or
refer to or consult with a pain management specialist.
3. Prescribe naloxone for any patient when risk factors of
prior overdose, substance [ abuse misuse ],
doses in excess of 120 MME/day, or concomitant benzodiazepine is present; and
4. Document the rationale to continue opioid therapy every
three months.
C. Buprenorphine [ may
mono-product in tablet form shall not ] be prescribed [ or
administered ] for chronic pain [ in formulation
and dosages that are FDA-approved for that purpose ].
D. Due to a higher risk of fatal overdose when opioids,
including buprenorphine, are given with other opioids, benzodiazepines,
sedative hypnotics, carisoprodol, and tramadol, the prescriber shall only co-prescribe
these substances when there are extenuating circumstances and shall document in
the medical record a tapering plan to achieve the lowest possible effective
doses of these medications if prescribed.
E. The practitioner shall regularly evaluate for opioid
use disorder and shall initiate specific treatment for opioid use disorder,
consult with an appropriate health care provider, or refer the patient for
evaluation and treatment if indicated.
18VAC85-21-80. Treatment plan for chronic pain.
A. The medical record shall include a treatment plan that
states measures to be used to determine progress in treatment, including pain
relief and improved physical and psychosocial function, quality of life, and
daily activities.
B. The treatment plan shall include further diagnostic
evaluations and other treatment modalities or rehabilitation that may be
necessary depending on the etiology of the pain and the extent to which the
pain is associated with physical and psychosocial impairment.
C. The prescriber shall document in the medical record
the presence or absence of any indicators for medication [ abuse,
misuse ] or diversion and shall take appropriate action.
18VAC85-21-130. General provisions pertaining to prescribing
of buprenorphine for addiction treatment.
A. Practitioners engaged in office-based opioid addiction
treatment with buprenorphine shall have obtained a SAMHSA waiver and the
appropriate U.S. Drug Enforcement Administration registration.
B. Practitioners shall abide by all federal and state laws
and regulations governing the prescribing of buprenorphine for the treatment of
opioid use disorder.
C. Physician assistants and nurse practitioners who have
obtained a SAMHSA waiver shall only prescribe buprenorphine for opioid
addiction pursuant to a practice agreement with a waivered doctor of medicine
or doctor of osteopathic medicine.
D. Practitioners engaged in medication-assisted treatment
shall either provide counseling in their practice or refer the patient to a
mental health service provider, as defined in § 54.1-2400.1 of the Code of
Virginia, who has the education and experience to provide substance [ abuse
misuse ] counseling. The practitioner shall document provision of
counseling or referral in the medical record.
18VAC85-21-140. Patient assessment and treatment planning
for addiction treatment.
A. A practitioner shall perform and document an assessment
that includes a comprehensive medical and psychiatric history, substance
[ abuse misuse ] history, family history and
psychosocial supports, appropriate physical examination, urine drug screen,
pregnancy test for women of childbearing age and ability, a check of the
Prescription Monitoring Program, and, when clinically indicated, infectious
disease testing for human immunodeficiency virus, hepatitis B, hepatitis C, and
tuberculosis.
B. The treatment plan shall include the practitioner's
rationale for selecting medication-assisted treatment, patient education,
written informed consent, how counseling will be accomplished, and a signed
treatment agreement that outlines the responsibilities of the patient and the
prescriber.
18VAC85-21-150. Treatment with buprenorphine for addiction.
A. Buprenorphine without naloxone (buprenorphine
mono-product) shall not be prescribed except:
1. When a patient is pregnant;
2. When converting a patient from methadone or
buprenorphine mono-product to buprenorphine containing naloxone for a period
not to exceed seven days; [ or ]
3. In formulations other than tablet form for indications
approved by the FDA [ ; or
4. For patients who have a demonstrated intolerance to
naloxone, such prescriptions for the mono-product shall not exceed 3.0% of the
total prescriptions for buprenorphine written by the prescriber, and the
exception shall be clearly documented in the patient's medical record ].
B. Buprenorphine mono-product tablets may be administered
directly to patients in federally licensed opioid treatment programs. With the
exception of those conditions listed in subsection A of this section, only the
buprenorphine product containing naloxone shall be prescribed or
dispensed for use off site from the program.
C. The evidence for the decision to use buprenorphine
mono-product shall be fully documented in the medical record.
D. Due to a higher risk of fatal overdose when buprenorphine
is prescribed with other opioids, benzodiazepines, sedative hypnotics,
carisoprodol, and tramadol, the prescriber shall only co-prescribe these
substances when there are extenuating circumstances and shall document in the
medical record a tapering plan to achieve the lowest possible effective doses
if these medications are prescribed.
E. Prior to starting medication-assisted treatment, the
practitioner shall perform a check of the Prescription Monitoring Program.
F. During the induction phase, except for medically
indicated circumstances as documented in the medical record, patients should be
started on no more than eight milligrams of buprenorphine per day. The patient
shall be seen by the prescriber at least once a week.
G. During the stabilization phase, the prescriber shall
increase the daily dosage of buprenorphine in safe and effective increments to
achieve the lowest dose that avoids intoxication, withdrawal, or significant
drug craving.
H. Practitioners shall take steps to reduce the chances of
buprenorphine diversion by using the lowest effective dose, appropriate
frequency of office visits, pill counts, and checks of the Prescription
Monitoring Program. The practitioner shall also require urine drug screens or
serum medication levels at least every three months for the first year of
treatment and at least every six months thereafter.
I. Documentation of the rationale for prescribed doses
exceeding 16 milligrams of buprenorphine per day shall be placed in the medical
record. Dosages exceeding 24 milligrams of buprenorphine per day shall not be
prescribed.
J. The practitioner shall incorporate relapse prevention
strategies into counseling or assure that they are addressed by a mental health
service provider, as defined in § 54.1-2400.1 of the Code of Virginia, who has
the education and experience to provide substance [ abuse
misuse ] counseling.
18VAC85-21-160. Special populations in addiction treatment.
A. Pregnant women [ shall may ]
be treated with the buprenorphine mono-product, usually 16 milligrams per
day or less.
B. Patients younger than the age of 16 years shall not be
prescribed buprenorphine for addiction treatment unless such treatment is
approved by the FDA.
C. The progress of patients with chronic pain shall be
assessed by reduction of pain and functional objectives that can be identified,
quantified, and independently verified.
D. Practitioners shall (i) evaluate patients with medical
comorbidities by history, physical exam, appropriate laboratory studies and
(ii) be aware of interactions of buprenorphine with other prescribed
medications.
E. Practitioners shall not undertake buprenorphine
treatment with a patient who has psychiatric comorbidities and is not stable. A
patient who is determined by the prescriber to be psychiatrically unstable
shall be referred for psychiatric evaluation and treatment prior to initiating
medication-assisted treatment.
VA.R. Doc. No. R17-5033; Filed August 24, 2017,
TITLE 18. PROFESSIONAL AND OCCUPATIONAL LICENSING
BOARD OF NURSING
Emergency Regulation
Title of Regulation:
18VAC90-40. Regulations for Prescriptive Authority for Nurse Practitioners (amending 18VAC90-40-150, 18VAC90-40-160,
18VAC90-40-180, 18VAC90-40-190, 18VAC90-40-200, 18VAC90-40-250, 18VAC90-40-260,
18VAC90-40-270, 18VAC90-40-280).
Statutory Authority: §§ 54.1-2400 and 54.1-2928.2
of the Code of Virginia.
Effective Dates: August 24, 2017, through November 7,
2018.
Agency Contact: Jay P. Douglas, R.N., Executive
Director, Board of Nursing, 9960 Mayland Drive, Suite 300, Richmond, VA
23233-1463, telephone (804) 367-4520, FAX (804) 527-4455, or email
jay.douglas@dhp.virginia.gov.
Preamble:
Section 2.2-4011 of the Code of Virginia authorizes an
agency to adopt emergency regulations upon consultation with the Attorney
General, and the necessity for the action is at the sole discretion of the
Governor. Emergency regulations for nurse practitioners prescribing medication
containing opioids and products containing buprenorphine were published in 33:20 VA.R. 2265-2269 May 29, 2017,
and became effective May 8, 2017.
The Boards of Nursing and Medicine adopted amendments to
the emergency regulation that became effective May 8, 2017. The amendments (i)
prohibit prescribing buprenorphine mono-product in tablet form for chronic
pain; (ii) clarify the limitations on prescribing buprenorphine mono-product
for patients with a demonstrated intolerance to naloxone; (iii) permit
prescribing buprenorphine mono-product for a pregnant woman if medically
indicated; and (iv) replace the term "abuse" with "misuse."
The purpose of the amendments is to provide patients who
have a demonstrated intolerance to naloxone with access to buprenorphine in the
treatment of substance misuse and provide prescribers with definitive rules to
follow so that prescribers are more assured of their ability to treat pain in
an appropriate manner to avoid under-prescribing or over-prescribing.
18VAC90-40-150. Evaluation of the patient for acute pain.
A. The requirements of this part shall not apply to:
1. The treatment of acute pain related to (i) cancer, (ii)
a patient in hospice care, or (iii) a patient in palliative care;
2. The treatment of acute pain during an inpatient hospital
admission or in a nursing home or an assisted living facility that uses a sole
source pharmacy; or
3. A patient enrolled in a clinical trial as authorized by
state or federal law.
B. Nonpharmacologic and non-opioid treatment for pain
shall be given consideration prior to treatment with opioids. If an opioid is
considered necessary for the treatment of acute pain, the practitioner shall
give a short-acting opioid in the lowest effective dose for the fewest possible
days.
C. Prior to initiating treatment with a controlled
substance containing an opioid for a complaint of acute pain, the prescriber
shall perform a history and physical examination appropriate to the complaint,
query the Prescription Monitoring Program as set forth in § 54.1-2522.1 of
the Code of Virginia, and conduct an assessment of the patient's history and
risk of substance [ abuse misuse ] as a
part of the initial evaluation.
18VAC90-40-160. Treatment of acute pain with opioids.
A. Initiation of opioid treatment for patients with acute
pain shall be with short-acting opioids.
1. A prescriber providing treatment for a patient with
acute pain shall not prescribe a controlled substance containing an opioid in a
quantity that exceeds a seven-day supply as determined by the manufacturer's
directions for use, unless extenuating circumstances are clearly documented in
the medical record. This shall also apply to prescriptions of a controlled
substance containing an opioid upon discharge from an emergency department.
2. An opioid prescribed as part of treatment for a surgical
procedure shall be for no more than 14 consecutive days in accordance with
manufacturer's direction and within the immediate perioperative period, unless
extenuating circumstances are clearly documented in the medical record.
B. Initiation of opioid treatment for all patients shall
include the following:
1. The practitioner shall carefully consider and document
in the medical record the reasons to exceed 50 MME/day.
2. Prior to exceeding 120 MME/day, the practitioner shall
document in the medical record the reasonable justification for such doses or
refer to or consult with a pain management specialist.
3. Naloxone shall be prescribed for any patient when risk
factors of prior overdose, substance [ abuse misuse ],
doses in excess of 120 MME/day, or concomitant benzodiazepine are present.
C. Due to a higher risk of fatal overdose when opioids are
used with benzodiazepines, sedative hypnotics, carisoprodol, and tramadol, the
prescriber shall only co-prescribe these substances when there are extenuating
circumstances and shall document in the medical record a tapering plan to
achieve the lowest possible effective doses if these medications are
prescribed.
D. Buprenorphine is not indicated for acute pain in the
outpatient setting, except when a prescriber who has obtained a SAMHSA
waiver is treating pain in a patient whose primary diagnosis is the
disease of addiction.
18VAC90-40-180. Evaluation of the chronic pain patient.
A. The requirements of this part shall not apply to:
1. The treatment of chronic pain related to (i) cancer,
(ii) a patient in hospice care, or (iii) a patient in palliative care;
2. The treatment of chronic pain during an inpatient
hospital admission or in a nursing home or an assisted living facility that
uses a sole source pharmacy; or
3. A patient enrolled in a clinical trial as authorized by
state or federal law.
B. Prior to initiating management of chronic pain with a
controlled substance containing an opioid, a medical history and physical
examination, to include a mental status examination, shall be performed and
documented in the medical record, including:
1. The nature and intensity of the pain;
2. Current and past treatments for pain;
3. Underlying or coexisting diseases or conditions;
4. The effect of the pain on physical and psychological
function, quality of life, and activities of daily living;
5. Psychiatric, addiction, and substance [ abuse
misuse ] histories of the patient and any family history of
addiction or substance [ abuse misuse ];
6. A urine drug screen or serum medication level;
7. A query of the Prescription Monitoring Program as set
forth in § 54.1-2522.1 of the Code of Virginia;
8. An assessment of the patient's history and risk of
substance [ abuse misuse ]; and
9. A request for prior applicable records.
C. Prior to initiating opioid analgesia for chronic pain,
the practitioner shall discuss with the patient the known risks and benefits of
opioid therapy and the responsibilities of the patient during treatment to
include securely storing the drug and properly disposing of any unwanted or
unused drugs. The practitioner shall also discuss with the patient an exit
strategy for the discontinuation of opioids in the event they are not
effective.
18VAC90-40-190. Treatment of chronic pain with opioids.
A. Nonpharmacologic and non-opioid treatment for pain
shall be given consideration prior to treatment with opioids.
B. In initiating opioid treatment for all patients, the
practitioner shall:
1. Carefully consider and document in the medical record
the reasons to exceed 50 MME/day;
2. Prior to exceeding 120 MME/day, the practitioner shall
document in the medical record the reasonable justification for such doses or
refer to or consult with a pain management specialist;
3. Prescribe naloxone for any patient when risk factors of
prior overdose, substance [ abuse misuse ],
doses in excess of 120 MME/day, or concomitant benzodiazepine are present; and
4. Document the rationale to continue opioid therapy every
three months.
C. Buprenorphine [ may
mono-product in tablet form shall not ] be prescribed [ or
administered ] for chronic pain [ in formulation
and dosages that are FDA-approved for that purpose ].
D. Due to a higher risk of fatal overdose when opioids,
including buprenorphine, are given with other opioids, benzodiazepines,
sedative hypnotics, carisoprodol, and tramadol, the prescriber shall only
co-prescribe these substances when there are extenuating circumstances and
shall document in the medical record a tapering plan to achieve the lowest
possible effective doses if these medications are prescribed.
E. The practitioner shall regularly evaluate for opioid
use disorder and shall initiate specific treatment for opioid use disorder,
consult with an appropriate health care provider, or refer the patient for
evaluation for treatment if indicated.
18VAC90-40-200. Treatment plan for chronic pain.
A. The medical record shall include a treatment plan that
states measures to be used to determine progress in treatment, including pain
relief and improved physical and psychosocial function, quality of life, and
daily activities.
B. The treatment plan shall include further diagnostic
evaluations and other treatment modalities or rehabilitation that may be
necessary depending on the etiology of the pain and the extent to which the
pain is associated with physical and psychosocial impairment.
C. The prescriber shall record in the medical records the
presence or absence of any indicators for medication misuse [ ,
abuse, ] or diversion and take appropriate action.
18VAC90-40-250. General provisions.
A. Practitioners engaged in office-based opioid addiction
treatment with buprenorphine shall have obtained a waiver from SAMHSA and the
appropriate U.S. Drug Enforcement Administration registration.
B. Practitioners shall abide by all federal and state laws
and regulations governing the prescribing of buprenorphine for the treatment of
opioid use disorder.
C. Nurse practitioners who have obtained a SAMHSA waiver
shall only prescribe buprenorphine for opioid addiction pursuant to a practice
agreement with a SAMHSA-waivered doctor of medicine or doctor of osteopathic
medicine.
D. Practitioners engaged in medication-assisted treatment
shall either provide counseling in their practice or refer the patient to a
mental health service provider, as defined in § 54.1-2400.1 of the Code of
Virginia, who has the education and experience to provide substance [ abuse
misuse ] counseling. The practitioner shall document provision of
counseling or referral in the medical record.
18VAC90-40-260. Patient assessment and treatment planning.
A. A practitioner shall perform and document an assessment
that includes a comprehensive medical and psychiatric history, substance
[ abuse misuse ] history, family history and
psychosocial supports, appropriate physical examination, urine drug screen,
pregnancy test for women of childbearing age and ability, a check of the
Prescription Monitoring Program, and, when clinically indicated, infectious
disease testing for human immunodeficiency virus, hepatitis B, hepatitis C, and
tuberculosis.
B. The treatment plan shall include the practitioner's
rationale for selecting medication assisted treatment, patient education,
written informed consent, how counseling will be accomplished, and a signed
treatment agreement that outlines the responsibilities of the patient and the
practitioner.
18VAC90-40-270. Treatment with buprenorphine.
A. Buprenorphine without naloxone (buprenorphine
mono-product) shall not be prescribed except:
1. When a patient is pregnant;
2. When converting a patient from methadone or
buprenorphine mono-product to buprenorphine containing naloxone for a period
not to exceed seven days; [ or ]
3. In formulations other than tablet form for indications
approved by the FDA [ ; or
4. For patients who have a demonstrated intolerance to
naloxone, such prescriptions for the mono-product shall not exceed 3.0% of the
total prescriptions for buprenorphine written by the prescriber, and the
exception shall be clearly documented in the patient's medical record ].
B. Buprenorphine mono-product tablets may be administered
directly to patients in federally licensed opiate treatment programs. With the
exception of those conditions listed in subsection A of this section, only the
buprenorphine product containing naloxone shall be prescribed or
dispensed for use off site from the program.
C. The evidence for the decision to use buprenorphine
mono-product shall be fully documented in the medical record.
D. Due to a higher risk of fatal overdose when
buprenorphine is prescribed with other opioids, benzodiazepines, sedative
hypnotics, carisoprodol, and tramadol, the prescriber shall only co-prescribe
these substances when there are extenuating circumstances and shall document in
the medical record a tapering plan to achieve the lowest possible effective
doses if these medications are prescribed.
E. Prior to starting medication-assisted treatment, the
practitioner shall perform a check of the Prescription Monitoring Program.
F. During the induction phase, except for medically
indicated circumstances as documented in the medical record, patients should be
started on no more than eight milligrams of buprenorphine per day. The patient
shall be seen by the prescriber at least once a week.
G. During the stabilization phase, the prescriber shall
increase the daily dosage of buprenorphine in safe and effective increments to
achieve the lowest dose that avoids intoxication, withdrawal, or significant
drug craving.
H. Practitioners shall take steps to reduce the chances of
buprenorphine diversion by using the lowest effective dose, appropriate
frequency of office visits, pill counts, and checks of the Prescription
Monitoring Program. The practitioner shall also require urine drug screens or
serum medication levels at least every three months for the first year of
treatment and at least every six months thereafter.
I. Documentation of the rationale for prescribed doses
exceeding 16 milligrams of buprenorphine per day shall be placed in the medical
record. Dosages exceeding 24 milligrams of buprenorphine per day shall not
be prescribed.
J. The practitioner shall incorporate relapse prevention
strategies into counseling or assure that they are addressed by a mental health
service provider, as defined in § 54.1-2400.1 of the Code of Virginia, who
has the education and experience to provide substance abuse counseling.
18VAC90-40-280. Special populations.
A. Pregnant women [ shall may ]
be treated with the buprenorphine mono-product, usually 16 milligrams per
day or less.
B. Patients younger than the age of 16 years shall not be
prescribed buprenorphine for addiction treatment unless such treatment is
approved by the FDA.
C. The progress of patients with chronic pain shall be
assessed by reduction of pain and functional objectives that can be identified,
quantified, and independently verified.
D. Practitioners shall (i) evaluate patients with medical
comorbidities by history, physical exam, and appropriate laboratory studies and
(ii) be aware of interactions of buprenorphine with other prescribed
medications.
E. Practitioners shall not undertake buprenorphine
treatment with a patient who has psychiatric comorbidities and is not stable. A
patient who is determined by the practitioner to be psychiatrically unstable
shall be referred for psychiatric evaluation and treatment prior to initiating
medication-assisted treatment.
VA.R. Doc. No. R17-5096; Filed August 24, 2017, 4:42 p.m.
TITLE 20. PUBLIC UTILITIES AND TELECOMMUNICATIONS
STATE CORPORATION COMMISSION
Proposed Regulation
REGISTRAR'S NOTICE: The
State Corporation Commission is claiming an exemption from the Administrative
Process Act in accordance with § 2.2-4002 A 2 of the Code of Virginia,
which exempts courts, any agency of the Supreme Court, and any agency that by
the Constitution is expressly granted any of the powers of a court of record.
Title of Regulation: 20VAC5-315. Regulations
Governing Net Energy Metering (amending 20VAC5-315-10 through 20VAC5-315-40;
adding 20VAC5-315-75).
Statutory Authority: §§ 12.1-13 and 56-594 of the
Code of Virginia.
Public Hearing Information: A public hearing will be
held upon request.
Public Comment Deadline: October 31, 2017.
Agency Contact: Armando deLeon, Utilities Engineer,
Division of Public Utility Regulation, State Corporation Commission, P.O. Box
1197, Richmond, VA 23218, telephone (804) 371-9392, FAX (804) 371-9350, or
email armando.deleon@scc.virginia.gov.
Summary:
Pursuant to Chapters 565 and 581 of the 2017 Acts of
Assembly, the proposed amendments (i) add a definition of small agricultural
generator and provide for the interconnection of small agricultural generators
to utilities because, as of July 1, 2019, agricultural customer-generators may
no longer interconnect with electric cooperatives but must do so as small
agricultural generators under § 56-594.2 of the Code of Virginia.
AT RICHMOND, AUGUST 25, 2017
COMMONWEALTH OF VIRGINIA, ex
rel.
STATE CORPORATION COMMISSION
CASE NO. PUR-2017-00099
Ex Parte: In the matter of amending regulations
governing net energy metering
ORDER ESTABLISHING PROCEEDING
The Regulations Governing Net Energy Metering, 20VAC5-315-10
et seq. ("Net Energy Metering Rules"), adopted by the State
Corporation Commission ("Commission") pursuant to § 56-594 of
the Virginia Electric Utility Regulation Act, Chapter 23
(§ 56-576 et seq.) of Title 56 of the Code of Virginia
("Code"), establish the requirements for participation by an eligible
customer-generator in net energy metering in the Commonwealth of Virginia. The
Net Energy Metering Rules include conditions for interconnection and metering,
billing, and contract requirements between net metering customers, electric
distribution companies, and energy service providers.
Chapters 565 and 581 of the 2017 Acts of Assembly
("Chapters 565 and 581") amended § 56-594 of the Code by adding a new
§ 56-594.2 to add a definition of "small agricultural generator"
and to provide for the interconnection of such generator to utilities. In
addition, Chapters 565 and 581 provide that on and after July 1, 2019,
interconnection of eligible agricultural customer-generators under § 56-594
shall cease for electric cooperatives only, and such facilities shall
interconnect solely as small agricultural generators under § 56-594.2. The
current Net Energy Metering Rules thus must be revised to reflect the changes
set forth in Chapters 565 and 581.
NOW THE COMMISSION, upon consideration of the matter, is of
the opinion and finds that a proceeding should be established to amend the Net
Energy Metering Rules to provide for the interconnection of small agricultural
generators, as defined in the Code.
To initiate this proceeding, the Commission Staff has
prepared proposed rules ("Proposed Rules") which are appended to this
Order. We will direct that notice of the Proposed Rules be given to the public
and that interested persons be provided an opportunity to file written comments
on, propose modifications or supplements to, or request a hearing on the
Proposed Rules. We will further direct that each Virginia electric
distribution company within the meaning of 20VAC5-315-20 serve a copy of this
Order upon each of their respective net metering customers and file a
certificate of service. Individuals should be specific in their comments,
proposals, or supplements to the Proposed Rules and address only those issues
pertaining to the amendment of § 56-594 of the Code of Virginia pursuant
to Chapters 565 and 581. Issues outside the scope of implementing these
amendments will not be open for consideration.
Accordingly, IT IS ORDERED THAT:
(1) This case is docketed and assigned Case No.
PUR-2017-00099.
(2) The Commission's Division of Information Resources shall
forward a copy of this Order to the Registrar of Regulations for publication in
the Virginia Register of Regulations.
(3) On or before September 19, 2017, each Virginia electric
distribution company shall serve a copy of this Order upon each of their
respective net metering customers and file with the Commission a certificate of
service no later than October 3, 2017, consistent with the findings above.
(4) On or before October 31, 2017, any interested person may
comment on, propose modifications or supplements to, or request a hearing on
the Proposed Rules by filing an original and fifteen (15) copies of such
comments or requests with Joel H. Peck, Clerk, State Corporation Commission,
c/o Document Control Center, P.O. Box 2118, Richmond, Virginia 23218.
Interested persons desiring to submit comments or hearing requests
electronically may do so by following the instructions available at the
Commission's website: http://www.scc.virginia.gov/case.
Individuals shall be specific in their comments, proposals, or supplements to
the Proposed Rules and shall address only those issues pertaining to the
amendment of § 56-594 of the Code of Virginia pursuant to Chapters 565 and
581. Issues outside the scope of implementing this amendment will not be open
for consideration. Any request for hearing shall state with specificity
why the issues raised in the request for hearing cannot be adequately addressed
in written comments. If a sufficient request for hearing is not received, the
Commission may consider the matter and enter an order based upon the papers
filed herein. Interested parties shall refer in their comments or requests to
Case No. PUR-2017-00099.
(5) This matter is continued.
AN ATTESTED COPY hereof shall be sent by the Clerk of the
Commission to all persons on the official Service List in this matter. The
Service List is available from the Clerk of the State Corporation Commission,
c/o Document Control Center, 1300 East Main Street, First Floor, Tyler
Building, Richmond, Virginia 23219.
20VAC5-315-10. Applicability and scope.
These regulations are promulgated pursuant to the provisions
of §§ 56-594 and 56-594.2 of the Virginia Electric Utility
Regulation Act (§ 56-576 et seq. of the Code of Virginia). They establish
requirements intended to facilitate net energy metering for customers owning
and operating, or contracting with persons to own or operate, or both,
electrical generators that use specific types of renewable energy as the total
fuel source. These regulations will standardize the interconnection requirements
for such facilities and will govern the metering, billing, payment and contract
requirements between net metering customers, electric distribution companies
and energy service providers. Agricultural net metering customers are subject
to the same provisions as nonagricultural net metering customers unless
otherwise specified. On or after July 1, 2019, interconnection of eligible
agricultural customer-generators shall cease for member-owned electric
cooperatives only, and such facilities shall interconnect solely as small
agricultural generators. For member-owned electric cooperatives, agricultural
net metering customers whose agricultural renewable fuel generators were
interconnected before July 1, 2019, may continue to participate in net energy metering
for a period not to exceed 25 years from the date of their agricultural
renewable fuel generator's original interconnection.
The amendments regarding agricultural net metering apply
to customers of investor-owned electric utilities on July 1, 2014, and apply to
customers of electric cooperatives on July 1, 2015, as provided in the State
Corporation Commission's Order Adopting Regulations, Case No. PUE-2014-00003,
dated June 23, 2014, and published in the Virginia Register of Regulations,
Volume 30, Issue 23, July 14, 2014.
These regulations also establish requirements for the
interconnection of small agricultural generators. Small agricultural generators
or agricultural renewable fuel generators may elect to interconnect as a net
metering customer or as small agricultural generators pursuant to
20VAC5-315-75, but not both. Existing eligible agricultural renewable fuel
generators may elect to become small agricultural generators, but may not
revert to being an agricultural renewable fuel generator after such election.
20VAC5-315-20. Definitions.
The following words and terms when used in this chapter shall
have the following meanings unless the context clearly indicates otherwise:
"Agricultural business" means any sole
proprietorship, corporation, partnership, electing small business (Subchapter
S) corporation, or limited liability company engaged primarily in the
production and sale of plants and animals, products collected from plants and
animals, or plant and animal services that are useful to the public.
"Agricultural net metering customer" means a
customer that operates an electrical generating facility consisting of one or
more agricultural renewable fuel generators having an aggregate generation
capacity of not more than 500 kilowatts as part of an agricultural business
under a net metering service arrangement. An agricultural net metering customer
may be served by multiple meters of one utility that are located at separate
but contiguous sites and that may be aggregated into one account. This account
shall be served under the appropriate tariff.
"Agricultural renewable fuel generator" or
"agricultural renewable fuel generating facility" means one or more
electrical generators that:
1. Use as their sole energy source solar power, wind power, or
aerobic or anaerobic digester gas;
2. The agricultural net metering customer owns and operates,
or has contracted with other persons to own or operate, or both;
3. Are located on land owned or controlled by the agricultural
business;
4. Are connected to the agricultural net metering customer's
wiring on the agricultural net metering customer's side of the agricultural net
metering customer's interconnection with the distributor;
5. Are interconnected and operated in parallel with an
electric company's distribution facilities; and
6. Are used primarily to provide energy to metered accounts of
the agricultural business.
"Billing period" means, as to a particular
agricultural net metering customer or a net metering customer, the time period
between the two meter readings upon which the electric distribution company and
the energy service provider calculate the agricultural net metering customer's
or net metering customer's bills.
"Billing period credit" means, for a nontime-of-use
agricultural net metering customer or a nontime-of-use net metering customer,
the quantity of electricity generated and fed back into the electric grid by
the agricultural net metering customer's agricultural renewable fuel generator
or generators or by the net metering customer's renewable fuel generator or
generators in excess of the electricity supplied to the customer over the
billing period. For time-of-use agricultural net metering customers or
time-of-use net metering customers, billing period credits are determined
separately for each time-of-use tier.
"Contiguous sites" means a group of land parcels in
which each parcel shares at least one boundary point with at least one other
parcel in the group. Property whose surface is divided only by public
right-of-way is considered contiguous.
"Customer" means a net metering customer or an
agricultural net metering customer.
"Demand charge-based time-of-use tariff" means a
retail tariff for electric supply service that has two or more time-of-use
tiers for energy-based charges and an electricity supply demand (kilowatt)
charge.
"Electric distribution company" means the entity
that owns and/or or operates the distribution facilities
delivering electricity to the premises of an agricultural net metering customer
or a net metering customer.
"Energy service provider (supplier)" means the
entity providing electricity supply service, either tariffed or competitive
service, to an agricultural net metering customer or a net metering customer.
"Excess generation" means the amount of electrical
energy generated in excess of the electrical energy consumed by the
agricultural net metering customer or net metering customer over the course of
the net metering period. For time-of-use agricultural net metering customers or
net metering customers, excess generation is determined separately for each
time-of-use tier.
"Generator" or "generating facility"
means an electrical generating facility consisting of one or more renewable
fuel generators or one or more agricultural renewable fuel generators that meet
the criteria under the definition of "net metering customer" and
"agricultural net metering customer," respectively.
"Net metering customer" means a customer owning and
operating, or contracting with other persons to own or operate, or both, an
electrical generating facility consisting of one or more renewable fuel
generators having an aggregate generation capacity of not more than 20
kilowatts for residential customers and not more than one megawatt for
nonresidential customers. The generating facility shall be operated under a net
metering service arrangement.
"Net metering period" means each successive
12-month period beginning with the first meter reading date following the final
interconnection of an agricultural net metering customer or a net metering customer's
generating facility consisting of one or more agricultural renewable fuel
generators or one or more renewable fuel generators, respectively, with the
electric distribution company's distribution facilities.
"Net metering service" means providing retail
electric service to an agricultural net metering customer operating an
agricultural renewable fuel generating facility or a net metering customer
operating a renewable fuel generating facility and measuring the difference,
over the net metering period, between the electricity supplied to the customer
from the electric grid and the electricity generated and fed back to the
electric grid by the customer.
"Person" means any individual, sole proprietorship,
corporation, limited liability company, partnership, association, company,
business, trust, joint venture, or other private legal entity, the
Commonwealth, or any city, county, town, authority, or other political
subdivision of the Commonwealth.
"Renewable Energy Certificate" or "REC"
represents the renewable energy attributes associated with the production of
one megawatt-hour (MWh) of electrical energy by a generator.
"Renewable fuel generator" or "renewable fuel
generating facility" means one or more electrical generators that:
1. Use renewable energy, as defined by § 56-576 of the
Code of Virginia, as their total fuel source;
2. The net metering customer owns and operates, or has
contracted with other persons to own or operate, or both;
3. Are located on the net metering customer's premises and connected
to the net metering customer's wiring on the net metering customer's side of
its interconnection with the distributor;
4. Are interconnected pursuant to a net metering arrangement
and operated in parallel with the electric distribution company's distribution
facilities; and
5. Are intended primarily to offset all or part of the net
metering customer's own electricity requirements. The capacity of any
generating facility installed on or after July 1, 2015, shall not exceed the
expected annual energy consumption based on the previous 12 months of billing
history or an annualized calculation of billing history if 12 months of billing
history is not available.
"Small agricultural generating facility" means
an electrical generating facility that:
1. Has a capacity of not more than 1.5 megawatts and does
not exceed 150% of the customer's expected annual energy consumption based on
the previous 12 months of billing history or an annualized calculation of
billing history if 12 months of billing history is not available;
2. Uses as its total source of fuel renewable energy;
3. Is located on the customer's premises and is
interconnected with the utility's distribution system through a separate meter;
4. Is interconnected and operated in parallel with an
electric utility's distribution system but not transmission facilities;
5. Is designed so that the electricity generated is
expected to remain on the utility's distribution system; and
6. Is a qualifying small power production facility pursuant
to the Public Utility Regulatory Policies Act of 1978 (P.L. 95-617).
"Small agricultural generator" means a customer
that:
1. Is not an eligible agricultural customer-generator
pursuant to § 56-594 of the Code of Virginia;
2. Operates a small agricultural generating facility as
part of an agricultural business;
3. May be served by multiple meters that are located at
separate but contiguous sites;
4. May aggregate the electricity consumption measured by
the meters, solely for purposes of calculating 150% of the customer's expected
annual energy consumption but not for billing or retail service purposes,
provided that the same utility serves all of its meters;
5. Uses not more than 25% of the contiguous land owned or
controlled by the agricultural business for purposes of the renewable energy
generating facility; and
6. Provides the electric utility with a certification,
attested under oath, as to the amount of land being used for renewable
generation.
"Time-of-use customer" means an agricultural net
metering customer or net metering customer receiving retail electricity supply
service under a demand charge-based time-of-use tariff.
"Time-of-use period" means an interval of time over
which the energy (kilowatt-hour) rate charged to a time-of-use customer does
not change.
"Time-of-use tier" or "tier" means all
time-of-use periods given the same name (e.g., on-peak, off-peak, critical
peak, etc.) for the purpose of time-differentiating energy
(kilowatt-hour)-based charges. The rates associated with a particular tier may
vary by day and by season.
20VAC5-315-30. Company notification.
A. A prospective agricultural net metering customer or a
prospective net metering customer (hereinafter referred to as
"customer") shall submit a completed commission-approved notification
form to the electric distribution company and, if different from the electric
distribution company, to the energy service provider, according to the time
limits in this subsection. If the prospective customer has contracted with
another person to own or operate, or both, the generator or generators, then
the notice will include detailed, current, and accurate contract information
for the owner or operator, or both, including without limitation, the name and
title of one or more individuals responsible for the interconnection and
operation of the generator or generators, a telephone number, a physical street
address other than a post office box, a fax number, and an email address for
each such person.
1. A residential customer shall notify its supplier and
receive approval to interconnect prior to installation or adding capacity to an
electrical generating facility. The electric distribution company shall have 30
days from the date of notification to determine whether the requirements
contained in 20VAC5-315-40 have been met. The date of notification shall be
considered to be the third day following the mailing of the notification form
by the prospective customer.
2. A nonresidential customer shall notify its supplier and
receive approval to interconnect prior to installation or adding capacity to an
electrical generating facility. The electric distribution company shall have 60
days from the date of notification to determine whether the requirements
contained in 20VAC5-315-40 have been met. The date of notification shall be
considered to be the third day following the mailing of the notification form
by the prospective customer.
B. Thirty-one days after the date of notification for a
residential customer, and 61 days after the date of notification for a
nonresidential customer, the prospective customer may interconnect and begin
operation of the generating facility unless the electric distribution company
or the energy service provider requests a waiver of this requirement under the
provisions of 20VAC5-315-80 prior to the 31st or 61st day, respectively. In
cases where the electric distribution company or energy service provider
requests a waiver, a copy of the request for waiver must be mailed
simultaneously by the requesting party to the prospective customer and to the commission's
Division of Energy Public Utility Regulation.
C. The electric distribution company shall file with the
commission's Division of Energy Public Utility Regulation a copy
of each completed notification form within 30 days of final interconnection.
20VAC5-315-40. Conditions of interconnection.
A. A prospective customer may begin operation of the
generating facility on an interconnected basis when:
1. The customer has properly notified both the electric
distribution company and energy service provider (in accordance with
20VAC5-315-30) of the customer's intent to interconnect.
2. If required by the electric distribution company's tariff,
the customer has installed a lockable, electric distribution company
accessible, load breaking manual disconnect switch at each of the facility's
generators.
3. The licensed electrician who installs the customer's
generator or generators certifies, by signing the commission-approved
notification form, that any required manual disconnect switch or switches are
being installed properly and that the generator or generators have been
installed in accordance with the manufacturer's specifications as well as all
applicable provisions of the National Electrical Code. If the customer or
licensed Virginia Class A or B general contractor installs the customer's
generator or generators, the signed final electrical inspection can be used in
lieu of the licensed electrician's certification.
4. The vendor certifies, by signing the commission-approved
notification form, that the generator or generators being installed are
in compliance with the requirements established by Underwriters Laboratories or
other national testing laboratories in accordance with IEEE Standard 1547,
Standard for Interconnecting Distributed Resources with Electric Power Systems,
July 2003.
5. In the case of static inverter-connected generators with an
alternating current capacity in excess of 10 kilowatts, the customer has had
the inverter settings inspected by the electric distribution company. The
electric distribution company may impose a fee on the customer of no more than
$50 for each generator that requires this inspection.
6. In the case of nonstatic inverter-connected generators, the
customer has interconnected according to the electric distribution company's
interconnection guidelines and the electric distribution company has inspected
all protective equipment settings. The electric distribution company may impose
a fee on the customer of no more than $50 for each generator that requires this
inspection.
7. The following requirements shall be met before
interconnection may occur:
a. Electric distribution facilities and customer impact
limitations. A customer's generator shall not be permitted to interconnect to
distribution facilities if the interconnection would reasonably lead to damage
to any of the electric distribution company's facilities or would reasonably
lead to voltage regulation or power quality problems at other customer revenue
meters due to the incremental effect of the generator on the performance of the
electric distribution system, unless the customer reimburses the electric
distribution company for its cost to accommodate the interconnection, including
the reasonable cost of equipment required for the interconnection.
b. Secondary, service, and service entrance limitations. The
capacity of the generators at any one service location shall be less than the
capacity of the electric distribution company-owned secondary, service, and
service entrance cable connected to the point of interconnection, unless the
customer reimburses the electric distribution company for the reasonable cost
of equipment required for the interconnection.
c. Transformer loading limitations. A customer's generator
shall not have the ability to overload the electric distribution company's
transformer, or any transformer winding, beyond manufacturer or nameplate
ratings, unless the customer reimburses the electric distribution company for
the reasonable cost of equipment required for the interconnection.
d. Integration with electric distribution company facilities
grounding. The grounding scheme of each generator shall comply with IEEE 1547,
Standard for Interconnecting Distributed Resources with Electric Power Systems,
July 2003, and shall be consistent with the grounding scheme used by the
electric distribution company. If requested by a prospective customer, the
electric distribution company shall assist the prospective customer in
selecting a grounding scheme that coordinates with its distribution system.
e. Balance limitation. The generator or generators shall not
create a voltage imbalance of more than 3.0% at any other customer's revenue
meter if the electric distribution company transformer, with the secondary
connected to the point of interconnection, is a three-phase transformer, unless
the customer reimburses the electric distribution company for the reasonable
cost of equipment required for the interconnection.
B. A prospective customer shall not be allowed to
interconnect a generator if doing so will cause the total rated generating
alternating current capacity of all interconnected net metered generators, as
defined in 20VAC5-315-20, within that customer's electric distribution
company's Virginia service territory to exceed 1.0% of that company's Virginia
peak-load forecast for the previous year. In any case where a prospective
customer has submitted a notification form required by 20VAC5-315-30 and that
customer's interconnection would cause the total rated generating alternating
current capacity of all interconnected net metered generators, as defined in
20VAC5-315-20, within that electric distribution company's service territory to
exceed 1.0% of that company's Virginia peak-load forecast for the previous
year, the electric distribution company shall, at the time it becomes aware of
the fact, send written notification to the prospective customer and to the
commission's Division of Energy Public Utility Regulation that
the interconnection is not allowed. In addition, upon request from any
customer, the electric distribution company shall provide to the customer the
amount of capacity still available for interconnection pursuant to § 56-594 D
of the Code of Virginia.
C. Neither the electric distribution company nor the energy
service provider shall impose any charges upon a customer for any
interconnection requirements specified by this chapter, except as provided
under subdivisions A 5, A 6, and A 7 of this section,
20VAC5-315-50, and 20VAC5-315-70 as related to additional metering.
D. A customer shall immediately notify the electric
distribution company of any changes in the ownership of, operational
responsibility for, or contact information for any of the customer's
generators.
20VAC5-315-75. Interconnection of small agricultural
generators.
Small agricultural generators electing to interconnect
pursuant to this section shall enter into a power purchase agreement with its
supplier to sell all of the electricity generated from its small agricultural
generating facility. The customer's supplier shall be obligated by the power
purchase agreement to purchase the excess generation at a price equal to a rate
agreed upon by the parties that is not less than the utility's
commission-approved avoided cost tariff for energy and capacity.
Small agricultural generators with renewable energy
certificates or other environmental attributes generated by the small
agricultural generating facility shall have the rights described in
20VAC5-315-50.
Small agricultural generators shall abide by the small
generator interconnection process described in 20VAC5-314. Such customer shall
be responsible for all costs associated with any interconnection or engineering
studies that may be required prior to interconnection.
DOCUMENTS INCORPORATED BY REFERENCE
1547, IEEE Standard for Interconnecting Distributed Resources
with Electric Power Systems, July 2003, The Institute of Electrical and
Electronics Engineers, Inc.
Rider G, Renewable Energy Program, Virginia Electric and
Power Company, January 1, 2009.
Rider
G, Renewable Energy Program, Virginia Electric and Power Company, January 1,
2012
VA.R. Doc. No. R18-5222; Filed August 25, 2017, 12:31 p.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Fast-Track Regulation
Title of Regulation: 22VAC40-12. Public Participation Guidelines (amending 22VAC40-12-50).
Statutory Authority: §§ 2.2-4007.02 and 63.2-217 and of the Code of Virginia.
Public Hearing Information: No public hearings are scheduled.
Public Comment Deadline: October 18, 2017.
Effective Date: November 3, 2017.
Agency Contact: Karin Clark, Regulatory Coordinator, Department of Social Services, 801 East Main Street, Richmond, VA 23219, telephone (804) 726-7017, or email karin.clark@dss.virginia.gov.
Basis: Section 63.2-217 of the Code of Virginia authorizes the State Board of Social Services to adopt regulations necessary or desirable to carry out Title 63.2 of the Code of Virginia. Section 2.2-4007.02 of the Code of Virginia mandates each agency develop, adopt, and use public participation guidelines for soliciting the input of interested parties in the formation and development of its regulations.
Purpose: Public participation guidelines exist to promote public involvement in the development, amendment, or repeal of State Board of Social Services regulations. The State Board of Social Services promulgates regulations for programs that help to protect the safety and welfare of citizens. The goal of the proposal is to align the regulation with the Code of Virginia regarding citizen opportunity to utilize representation when submitting input on regulatory actions.
Rationale for Using Fast-Track Rulemaking Process: Executive Order 17 (2014) allows state agencies to use a fast-track rulemaking process to expedite regulatory changes that are expected to be noncontroversial. This regulatory action reflects opportunities for citizen input into the regulatory process as already established in the Code of Virginia. No controversy it anticipated.
Substance: Currently 22VAC40-12-50 affords interested persons the opportunity to submit data, views, and arguments related to regulatory actions, but makes no reference to affording the opportunity to be accompanied by or represented by counsel or other representatives. The amendment adds this provision.
Issues: The primary advantage to the public and Commonwealth is consistency between Code of Virginia and regulatory provisions regarding citizen input. Interested persons will be aware of these provisions whether they access the Code of Virginia or 22VAC40-12. There are no disadvantages to the public and Commonwealth.
Small Business Impact Review Report of Findings: This fast-track regulatory action serves as the report of the findings of the regulatory review pursuant to § 2.2-4007.1 of the Code of Virginia.
Department of Planning and Budget's Economic Impact Analysis:
Summary of the Proposed Amendments to Regulation. Pursuant to Chapter 795 of the 2012 Acts of Assembly,1 the State Board of Social Services (Board) proposes to specify in this regulation that interested persons shall be afforded an opportunity to be accompanied by and represented by counsel or other representative when submitting data, views, and arguments, either orally or in writing, to the agency regarding the formation and development of a regulation.
Result of Analysis. The benefits likely exceed the costs for all proposed changes.
Estimated Economic Impact. The current Public Participation Guidelines state that: "In considering any nonemergency, nonexempt regulatory action, the agency shall afford interested persons an opportunity to submit data, views, and arguments, either orally or in writing, to the agency." The Board proposes to append "and (ii) be accompanied by and represented by counsel or other representative."
Chapter 795 of the 2012 Acts of Assembly added to § 2.2-4007.02 of the Code of Virginia "Public participation guidelines" that interested persons also be afforded an opportunity to be accompanied by and represented by counsel or other representative. Since the Code of Virginia already specifies that interested persons shall be afforded an opportunity to be accompanied by and represented by counsel or other representative, the Board's proposal to add this language to the regulation will not change the law in effect, but will be beneficial in that it will inform interested parties who read this regulation but not the statute of their legal rights concerning representation.
Businesses and Entities Affected. The proposed amendment potentially affects all individuals who comment on pending regulatory changes.
Localities Particularly Affected. The proposed amendment does not disproportionately affect particular localities.
Projected Impact on Employment. The proposed amendment does not significantly affect employment.
Effects on the Use and Value of Private Property. The proposed amendment does not affect the use and value of private property.
Real Estate Development Costs. The proposed amendment does not affect real estate development costs.
Small Businesses:
Definition. Pursuant to § 2.2-4007.04 of the Code of Virginia, small business is defined as "a business entity, including its affiliates, that (i) is independently owned and operated and (ii) employs fewer than 500 full-time employees or has gross annual sales of less than $6 million."
Costs and Other Effects. The proposed amendment does not affect costs for small businesses.
Alternative Method that Minimizes Adverse Impact. The proposed amendment does not adversely affect small businesses.
Adverse Impacts:
Businesses. The proposed amendment does not adversely affect businesses.
Localities. The proposed amendment does not adversely affect localities.
Other Entities. The proposed amendment does not adversely affect other entities.
________________________
1 See http://leg1.state.va.us/cgi-bin/legp504.exe?121+ful+CHAP0795+hil
Agency's Response to Economic Impact Analysis: The Department of Social Services reviewed the economic impact analysis prepared by the Department of Planning and Budget and concurs.
Summary:
Pursuant to § 2.2-4007.02 of the Code of Virginia, the amendment provides that interested persons submitting data, views, and arguments on a regulatory action may be accompanied by and represented by counsel or another representative.
Part III
Public Participation Procedures
22VAC40-12-50. Public comment.
A. In considering any nonemergency, nonexempt regulatory action, the agency shall afford interested persons an opportunity to (i) submit data, views, and arguments, either orally or in writing, to the agency; and (ii) be accompanied by and represented by counsel or other representative. Such opportunity to comment shall include an online public comment forum on the Town Hall.
1. To any requesting person, the agency shall provide copies of the statement of basis, purpose, substance, and issues; the economic impact analysis of the proposed or fast-track regulatory action; and the agency's response to public comments received.
2. The agency may begin crafting a regulatory action prior to or during any opportunities it provides to the public to submit comments.
B. The agency shall accept public comments in writing after the publication of a regulatory action in the Virginia Register as follows:
1. For a minimum of 30 calendar days following the publication of the notice of intended regulatory action (NOIRA).
2. For a minimum of 60 calendar days following the publication of a proposed regulation.
3. For a minimum of 30 calendar days following the publication of a reproposed regulation.
4. For a minimum of 30 calendar days following the publication of a final adopted regulation.
5. For a minimum of 30 calendar days following the publication of a fast-track regulation.
6. For a minimum of 21 calendar days following the publication of a notice of periodic review.
7. Not later than 21 calendar days following the publication of a petition for rulemaking.
C. The agency may determine if any of the comment periods listed in subsection B of this section shall be extended.
D. If the Governor finds that one or more changes with substantial impact have been made to a proposed regulation, he may require the agency to provide an additional 30 calendar days to solicit additional public comment on the changes in accordance with § 2.2-4013 C of the Code of Virginia.
E. The agency shall send a draft of the agency's summary description of public comment to all public commenters on the proposed regulation at least five days before final adoption of the regulation pursuant to § 2.2-4012 E of the Code of Virginia.
VA.R. Doc. No. R18-5137; Filed August 24, 2017, 12:45 p.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
REGISTRAR'S NOTICE: The State Board of Social Services is claiming an exemption from Article 2 of the Administrative Process Act in accordance with § 2.2-4006 A 4 a of the Code of Virginia, which excludes regulations that are necessary to conform to changes in Virginia statutory law or the appropriation act where no agency discretion is involved. The State Board of Social Services will receive, consider, and respond to petitions by any interested person at any time with respect to reconsideration or revision.
Title of Regulation: 22VAC40-80. General Procedures and Information for Licensure (amending 22VAC40-80-10, 22VAC40-80-340).
Statutory Authority: §§ 63.2-217, 63.2-1732, 63.2-1733, and 63.2-1734 of the Code of Virginia.
Effective Date: October 19, 2017.
Agency Contact: Cheryl H. Morris, Licensing Program Consultant, Department of Social Services, 801 East Main Street, Richmond, VA 23219, telephone (540) 347-6375, FAX (540) 347-6304, or email cheryl.morris@dss.virginia.gov.
Summary:
The amendments (i) conform the definitions of "applicant" and "licensee" to the definition of "person who operates or maintains a child welfare agency" under § 63.2-1701 of the Code of Virginia, pursuant to Chapter 196 of the 2017 Acts of Assembly, to include an individual; corporation; partnership; association; limited liability company; local government; state agency, including any department, institution, authority, instrumentality, board, or other administrative agency of the Commonwealth; or other legal or commercial entity that operates or maintains a child welfare agency; and (ii) change the timeframe for the aggregate amount of civil penalties for noncompliance by assisted living facilities to not exceed $10,000 in a 24-month period to a 12-month period pursuant to Chapters 138 and 283 of the 2017 Acts of Assembly.
Part I
Introduction
22VAC40-80-10. Definitions.
The following words and terms when used in this chapter shall have the following meanings unless the context clearly indicates otherwise:
"Administrative hearing" means a hearing that is conducted pursuant to § 2.2-4020 of the Administrative Process Act.
"Adult care facility" means a licensed assisted living facility or adult day care center.
"Adverse action" means any case where the department either gives notice of revocation or refuses to issue a license for an assisted living facility, adult day care center or child welfare agency or imposes another administrative sanction pursuant to § 63.2-1709 of the Code of Virginia.
"Aggrieved party" means an applicant or licensee who has requested an appeal in accordance with instructions provided after the department has given written notice of the imposition of an administrative sanction or adverse action for an assisted living facility, adult day care center, or child welfare agency.
"Allowable variance" means permission is granted by the department to a licensee or applicant for licensure to meet the intent of a standard by some means other than as specified by the standard when the applicant or licensee has demonstrated that (i) the implementation of a standard would impose a substantial financial or programmatic hardship and (ii) the variance would not adversely affect the safety and well-being of persons in care.
"Applicant" means the person, corporation, partnership, association, limited liability company, or public agency that has applied for a license to operate or maintain an assisted living facility, adult day care center, or child welfare agency. For a child welfare agency, the "person who operates or maintains a child welfare agency" means any individual; corporation; partnership; association; limited liability company; local government; state agency, including any department, institution, authority, instrumentality, board, or other administrative agency of the Commonwealth; or other legal or commercial entity that operates or maintains a child welfare agency.
"Board" means the State Board of Social Services.
"Child welfare agency" means a child day center, child-placing agency, child-caring institution, family day home, family day system, or independent foster home.
"Commissioner" means the Commissioner of the Department of Social Services.
"Complaint" means an accusation that a facility that is subject to licensure is operating without a license or that a licensed facility is not in compliance with licensing standards or law.
"Conditional license" means a license that may be issued to a new facility to operate in order to permit the applicant to demonstrate compliance with specified standards.
"Consent agreement" means an agreement between the licensee and the department that the licensee will perform specific actions for the purpose of correcting violations to come into compliance with standards or statutes.
"Council" means the Child Day-Care Council.
"Day" means a calendar day unless otherwise specified.
"Denial" means the act of refusing to grant a license after receipt of an initial or renewal application.
"Department" means the Department of Social Services.
"Early compliance" means that the licensee has demonstrated full compliance with requirements, allowing the department to replace a provisional or conditional license with a regular license.
"Functional design" means the design features of building and grounds not regulated by the Building Code, necessary for particular activities and operations of a facility subject to licensure by the Department of Social Services.
"Good character and reputation" means findings have been established and knowledgeable, reasonable, and objective people agree that the individual (i) maintains business or professional, family, and community relationships that are characterized by honesty, fairness, truthfulness, and dependability; and (ii) has a history or pattern of behavior that demonstrates the individual is suitable and able to administer a program for the care, supervision, and protection of children or adults. Relatives by blood or marriage and persons who are not knowledgeable of the individual, such as recent acquaintances, may not be considered objective references.
"Hearing" means agency processes other than those informational or factual inquiries of an informal nature provided in §§ 2.2-4007 and 2.2-4019 of the Code of Virginia and includes only (i) opportunity for private parties to submit factual proofs in formal proceedings as provided in § 2.2-4009 of the Code of Virginia in connection with the making of regulations or (ii) a similar right of private parties of public agencies as provided in § 2.2-4020 of the Code of Virginia in connection with case decisions.
"Hearing coordinator" means the person designated by the Department of Social Services to perform certain administrative functions involved in setting up and carrying out the hearings concerning adverse action on a license for an assisted living facility, adult day care center or child welfare agency, as set out herein.
"Hearing officer" means an attorney selected from a list maintained by the Executive Secretary of the Supreme Court in accordance with § 2.2-4024 of the Code of Virginia to preside at hearings concerning adverse action on a license for an assisted living facility, adult day care center or child welfare agency.
"Informal conference" means the informal fact-finding procedures available pursuant to §§ 2.2-4019 and 2.2-3021 of the Code of Virginia.
"Licensee" means the person, corporation, partnership, association, limited liability company, or public agency to whom a license is issued and who is legally responsible for compliance with the regulations and statutory requirements related to the facility operation or maintenance of the assisted living facility, adult day care center, or child welfare agency. For a child welfare agency, the "person who operates or maintains a child welfare agency" means any individual; corporation; partnership; association; limited liability company; local government; state agency, including any department, institution, authority, instrumentality, board, or other administrative agency of the Commonwealth; or other legal or commercial entity that operates or maintains a child welfare agency.
"Probationary status" means placing a licensee on notice that the facility or agency is substantially out of compliance with the terms of its license and the health, safety, and well-being of persons in care are at risk. Probationary status is a precursor to more serious action such as revocation, denial, or injunctive action unless immediate corrective action occurs.
"Provisional license" means a license that may be issued upon expiration of a regular license when the licensee is temporarily unable to substantially comply with the requirements of the law and regulations.
"Recommended findings of fact and recommended decision" means the report prepared by the hearing officer upon evidence presented in the administrative hearing based on the applicable laws and regulations under which the department operates.
"Regular license" means a license that is issued for 12 months or more as provided in Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia to a facility determined to be in substantial compliance with applicable standards and regulations. The actual duration of the licensure period is stated on the license.
"Revocation" means the act of terminating a license during its effective dates because of findings of serious noncompliance.
"Special order" means an order imposing an administrative sanction issued to any party licensed pursuant to Title 63.2 of the Code of Virginia by the commissioner that has a stated duration of not more than 12 months. A special order shall be considered a case decision as defined in § 2.2-4001 of the Code of Virginia. The 12-month period begins 30 days after notification of the issuance of a special order or at the conclusion of all appeal steps.
"Substantial compliance" means that while there may be noncompliance with one or more standards that represents minimal risk, compliance clearly and obviously exists with most of the standards as a whole.
22VAC40-80-340. Administrative sanctions.
The commissioner may impose administrative sanctions or initiate court proceedings, severally or jointly, when appropriate in order to ensure prompt correction of violations involving noncompliance with state law or regulation in assisted living facilities, adult day care centers, and child welfare agencies as discovered through any inspection or investigation conducted by the Department of Social Services, the Virginia Department of Health, the Virginia Department of Mental Behavioral Health, Mental Retardation and Substance Abuse Developmental Services, or by state and local building or fire prevention officials. These administrative sanctions include:
1. Petitioning the court to appoint a receiver for any assisted living facility or adult day care center;
2. Revoking or denying renewal of a license for any assisted living facility or adult day care center that fails to comply with the limitations and standards set forth in its license for violation that adversely affects, or is an imminent and substantial threat to, the health, safety, or welfare of residents, or for permitting, aiding, or abetting the commission of any illegal act in an adult care facility;
3. Revoking or denying renewal of a license for any child welfare agency that fails to comply with the limitations and standards set forth in its license;
4. Requiring an assisted living facility to contract with an individual licensed by the Board of Long-Term Care Administrators to administer, manage, or operate the facility on an interim basis if the commissioner receives information from any source indicating imminent and substantial risk of harm to residents. This action shall be an attempt to bring the facility into compliance with all relevant requirements of law, regulation, or any plan of correction approved by the commissioner. The contract shall be negotiated in accordance with the provisions of § 63.2-1709 of the Code of Virginia;
5. Issuing a summary order of suspension of the license to operate an assisted living facility pursuant to proceedings set forth in § 63.2-1709 C of the Code of Virginia in conjunction with any proceedings for revocation, denial, or other action when conditions or practices exist that pose an imminent and substantial threat to the health, safety, and welfare of residents; and
6. Imposing administrative sanctions through the issuance of a special order as provided in § 63.2-1709.2 of the Code of Virginia. These include:
a. Placing a licensee on probation upon finding that the licensee is substantially out of compliance with the terms of the license and that the health and safety of residents, participants, or children are at risk;
b. Reducing the licensed capacity or prohibiting new admissions when the commissioner has determined that the licensee cannot make necessary corrections to achieve compliance with the regulations except by a temporary restriction of its scope of service;
c. Mandating training for the licensee or licensee's employees, with any costs to be borne by the licensee, when the commissioner has determined that the lack of such training has led directly to violations of regulations;
d. Assessing civil penalties of not more than $500 per inspection upon finding that the licensee of an adult day care center or child welfare agency is substantially out of compliance with the terms of its license and the health and safety of residents, participants, or children are at risk;
e. Assessing a civil penalty for each day an assisted living facility is or was out of compliance with the terms of its license and the health, safety, and welfare of residents are at risk. The aggregate amount of such civil penalties shall not exceed $10,000 in any 24-month 12-month period. Criteria for imposition of civil penalties and amounts, expressed in ranges, are developed by the board and are based upon the severity, pervasiveness, duration, and degree of risk to the health, safety, or welfare of residents. Such civil penalties shall be applied by the commissioner in a consistent manner;
f. Requiring licensees to contact parents, guardians, or other responsible persons in writing regarding health and safety violations; and
g. Preventing licensees who are substantially out of compliance with the licensure terms or in violation of the regulations from receiving public funds.
VA.R. Doc. No. R18-5163; Filed August 18, 2017, 10:12 a.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
REGISTRAR'S NOTICE: The
State Board of Social Services is claiming an exemption from Article 2 of the
Administrative Process Act in accordance with § 2.2-4006 A 4 a of the Code of
Virginia, which excludes regulations that are necessary to conform to changes
in Virginia statutory law or the appropriation act where no agency discretion
is involved. The State Board of Social Services will receive, consider, and
respond to petitions by any interested person at any time with respect to
reconsideration or revision.
Title of Regulation: 22VAC40-90. Regulation for
Background Checks for Assisted Living Facilities and Adult Day Care Centers (amending 22VAC40-90-10, 22VAC40-90-70).
Statutory Authority: §§ 63.2-217, 63.2-1702, 63.2-1720,
and 63.2-1721 of the Code of Virginia.
Effective Date: October 19, 2017.
Agency Contact: Judith McGreal, Department of Social
Services, 801 East Main Street, Richmond, VA 23219, telephone (804) 726-7157,
FAX (804) 726-7132, or email judith.mcgreal@dss.virginia.gov.
Summary:
The amendments (i) conform the definition of "barrier
crimes" to statutory changes made pursuant to Chapters 201 and 809 of the
2017 Acts of Assembly, which moved the listing of barrier crimes for assisted
living facilities and adult day care centers from § 63.2-1719 to § 19.2-392.02
of the Code of Virginia and expanded the list to include additional crimes;
(ii) allow assisted living facilities and adult day care centers to continue to
employ a person convicted of one misdemeanor barrier crime not involving abuse or
neglect if five years has elapsed following the conviction; and (iii) update a
cross reference in 22VAC40-90-70.
Part I
Introduction
22VAC40-90-10. Definitions.
The following words and terms when used in conjunction with
this chapter shall have the following meanings:
"Applicant for licensure" means the entity applying
for approval as a licensed assisted living facility. An applicant may be an
individual, association, partnership, limited liability company, corporation or
public agency.
"Barrier crimes" means certain crimes that
automatically bar individuals convicted of same from employment at a licensed
assisted living facility or adult day care center and that automatically bar
licensure of applicants convicted of same from assisted living facility
licensure. These crimes, as specified by § 63.2-1719 § 19.2-392.02
of the Code of Virginia, are felony violations of a protective order as set
out in § 16.1-253.2; murder or manslaughter as set out in Article 1
(§ 18.2-30 et seq.) of Chapter 4 of Title 18.2; malicious wounding by mob
as set out in § 18.2-41; abduction as set out in subsection A or B of
§ 18.2-47; abduction for immoral purposes as set out in § 18.2-48;
assaults and bodily woundings as set out in Article 4 (§ 18.2-51 et seq.)
of Chapter 4 of Title 18.2; robbery as set out in § 18.2-58; carjacking as
set out in § 18.2-58.1; extortion by threat as set out in § 18.2-59;
threats of death or bodily injury as set out in § 18.2-60; felony stalking
as set out in § 18.2-60.3; felony violation of a protective order as set
out in § 18.2-60.4; sexual assault as set out in Article 7 (§ 18.2-61
et seq.) of Chapter 4 of Title 18.2; arson as set out in Article 1
(§ 18.2-77 et seq.) of Chapter 5 of Title 18.2; drive-by shooting as set
out in § 18.2-286.1; use of a machine gun in a crime of violence as set
out in § 18.2-289; aggressive use of a machine gun as set out in
§ 18.2-290; use of a sawed-off shotgun in a crime of violence as set out
in subsection A of § 18.2-300; pandering as set out in § 18.2-355;
crimes against nature involving children as set out in § 18.2-361; incest
as set out in § 18.2-366; taking indecent liberties with children as set out
in § 18.2-370 or § 18.2-370.1; abuse and neglect of children as set
out in § 18.2-371.1; failure to secure medical attention for an injured
child as set out in § 18.2-314; obscenity offenses as set out in
§ 18.2-374.1; possession of child pornography as set out in
§ 18.2-374.1:1; electronic facilitation of pornography as set out in
§ 18.2-374.3; abuse and neglect of incapacitated adults as set out in
§ 18.2-369; employing or permitting a minor to assist in an act
constituting an offense under Article 5 (§ 18.2-372 et seq.) of Chapter 8
of Title 18.2 as set out in § 18.2-379; delivery of drugs to prisoners as
set out in § 18.2-474.1; escape from jail as set out in § 18.2-477;
felonies by prisoners as set out in § 53.1-203; or an equivalent offense
in another state a felony violation of § 16.1-253.2; any violation
of § 18.2-31, 18.2-32, 18.2-32.1, 18.2-32.2, 18.2-33, 18.2-35,
18.2-36, 18.2-36.1, 18.2-36.2, 18.2-41, or 18.2-42; any felony violation of
§ 18.2-46.2, 18.2-46.3, 18.2-46.3:1, or 18.2-46.3:3; any violation of
§ 18.2-46.5, 18.2-46.6, or 18.2-46.7; any violation of subsection A or B
of § 18.2-47; any violation of § 18.2-48, 18.2-49, or 18.2-50.3; any
violation of § 18.2-51, 18.2-51.1, 18.2-51.2, 18.2-51.3, 18.2-51.4,
18.2-51.5, 18.2-51.6, 18.2-52, 18.2-52.1, 18.2-53, 18.2-53.1, 18.2-54.1,
18.2-54.2, 18.2-55, 18.2-55.1, 18.2-56, 18.2-56.1, 18.2-56.2, 18.2-57,
18.2-57.01, 18.2-57.02, 18.2-57.2, 18.2-58, 18.2-58.1, 18.2-59, 18.2-60, or
18.2-60.1; any felony violation of § 18.2-60.3 or 18.2-60.4; any violation
of § 18.2-61, 18.2-63, 18.2-64.1, 18.2-64.2, 18.2-67.1, 18.2-67.2,
18.2-67.3, 18.2-67.4, 18.2-67.4:1, 18.2-67.4:2, 18.2-67.5, 18.2-67.5:1,
18.2-67.5:2, 18.2-67.5:3, 18.2-77, 18.2-79, 18.2-80, 18.2-81, 18.2-82, 18.2-83,
18.2-84, 18.2-85, 18.2-86, 18.2-87, 18.2-87.1, or 18.2-88; any felony violation
of § 18.2-279, 18.2-280, 18.2-281, 18.2-282, 18.2-282.1, 18.2-286.1, or
18.2-287.2; any violation of § 18.2-289, 18.2-290, 18.2-300, 18.2-308.4,
or 18.2-314; any felony violation of § 18.2-346; any violation of § 18.2-355,
18.2-356, 18.2-357, or 18.2-357.1; any violation of subsection B of
§ 18.2-361; any violation of § 18.2-366, 18.2-369, 18.2-370,
18.2-370.1, 18.2-370.2, 18.2-370.3, 18.2-370.4, 18.2-370.5, 18.2-370.6,
18.2-371.1, 18.2-374.1, 18.2-374.1:1, 18.2-374.3, 18.2-374.4, 18.2-379,
18.2-386.1, or 18.2-386.2; any felony violation of § 18.2-405 or 18.2-406;
any violation of § 18.2-408, 18.2-413, 18.2-414, 18.2-423, 18.2-423.01,
18.2-423.1, 18.2-423.2, 18.2-433.2, 18.2-472.1, 18.2-474.1, 18.2-477,
18.2-477.1, 18.2-477.2, 18.2-478, 18.2-479, 18.2-480, 18.2-481, 18.2-484,
18.2-485, 37.2-917, or 53.1-203; or any substantially similar offense under the
laws of another jurisdiction. Applicants for employment A
licensed assisted living facility or adult day care center may hire an applicant
or continue to employ a person convicted of one misdemeanor barrier crime
not involving abuse or neglect may be hired, or any substantially
similar offense under the laws of another jurisdiction, if five years have
elapsed following the conviction.
"Central Criminal Records Exchange" means the
information system containing conviction data of those crimes committed in
Virginia, maintained by the Department of State Police, through which the
criminal history record request form is processed.
"Criminal history record request" means the
Department of State Police form used to authorize the State Police to generate
a criminal history record report on an individual.
"Criminal history record report" means either the
criminal record clearance or the criminal history record issued by the Central
Criminal Records Exchange, Department of State Police. The criminal record
clearance provides conviction data only related to barrier crimes; the criminal
history record discloses all known conviction data.
"Employee" means compensated personnel working at a
facility regardless of role, service, age, function or duration of employment
at the facility. Employee also includes those individuals hired through a
contract to provide services for the facility.
"Facility" means an assisted living facility or
adult day care center subject to licensure by the Department of Social
Services.
"Sworn statement or affirmation" means a document
to be completed, signed, and submitted for licensure or employment. The
document discloses the licensure applicant's or employment applicant's criminal
convictions and pending criminal charges that occurred within or outside the
Commonwealth of Virginia. For applicants for licensure as an assisted living
facility, the document also discloses whether or not the applicant has been the
subject of a founded complaint of child abuse or neglect within or outside the
Commonwealth of Virginia. This is required as specified in §§ 63.2-1720
and 63.2-1721 of the Code of Virginia.
22VAC40-90-70. Issuance of a license.
The commissioner shall not issue a license to an assisted
living facility if an applicant for licensure required to have a background
check has any offense as defined in § 63.2-1719 of the Code of Virginia been
convicted of a barrier crime as defined in this chapter.
VA.R. Doc. No. R18-5161; Filed August 18, 2017, 10:10 a.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
REGISTRAR'S NOTICE: The State Board of Social Services is claiming an exemption from Article 2 of the Administrative Process Act in accordance with § 2.2-4006 A 4 a of the Code of Virginia, which excludes regulations that are necessary to conform to changes in Virginia statutory law or the appropriation act where no agency discretion is involved. The State Board of Social Services will receive, consider, and respond to petitions by any interested person at any time with respect to reconsideration or revision.
Title of Regulation: 22VAC40-120. Minimum Standards for Licensed Family Day-Care Systems (amending 22VAC40-120-10).
Statutory Authority: § 63.2-217 of the Code of Virginia.
Effective Date: October 19, 2017.
Agency Contact: Deborah Eves, Licensing Program Consultant, Department of Social Services, 801 East Main Street, Richmond, VA 23219, telephone (804) 726-7506, FAX (804) 726-7132, or email deborah.eves@dss.virginia.gov.
Summary:
The amendment conforms the definition of "person" with the definition of "person who operates or maintains a child welfare agency" under § 63.2-1701 of the Code of Virginia, pursuant to Chapter 196 of the 2017 Acts of Assembly, to include an individual; corporation; partnership; association; limited liability company; local government; state agency, including any department, institution, authority, instrumentality, board, or other administrative agency of the Commonwealth; or other legal or commercial entity that operates or maintains a child welfare agency.
22VAC40-120-10. Definitions; license provisions.
A. Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia sets forth the responsibility of the Department of Social Services for licensure of family day-care systems, including the authority and responsibility of the State Board of Social Services for the development of regulations containing minimum standards and requirements.
It is a misdemeanor to operate a family day-care system without a license. (§ 63.2-1712 of the Code of Virginia)
B. Definitions. The following words and terms, when used in this chapter, shall have the following meanings unless the context clearly indicates otherwise:
"Abused or neglected child" (see § 63.2-100 of the Code of Virginia) means any child younger than 18 years of age whose parents or other persons responsible for his or her care:
a. Create or inflict, threaten to create or inflict, or allow to be created or inflicted a physical or mental injury by other than accidental means, or create a substantial risk of death, disfigurement, or impairment of bodily or mental functions;
b. Neglect or refuse to provide care necessary for the child's health, unless the child is, in good faith, under treatment solely by spiritual means through prayer, according to the practice of a recognized church or denomination;
c. Abandon the child;
d. Commit or allow to be committed any sexual act upon a child in violation of the law.
"Child" means any person younger than 18 years of age.
"Commissioner" means the Commissioner of Social Services also known as the Director of the Virginia Department of Social Services. (§ 63.2-100 of the Code of Virginia)
"Complaint" means an accusation received either orally or in writing that:
a. A licensed family day-care system is not in compliance with one or more of these standards or one or more statutory requirements; or
b. A family day-care system home is not in compliance with one or more applicable requirements of these standards or one or more requirements as established by the family day-care system; or
c. A child or children in the care of a family day-care home, which is a member of a licensed family day-care system, is or are being abused or neglected.
"Day-care" means care, protection, and guidance provided to a child or group of children separated from their parents or guardian for less than 24 hours per day at a location other than the home of the parents or guardian.
"Day-care provider" means an individual who, by contract with a family day-care system, provides day-care in his home.
"Department" means the Virginia Department of Social Services.
"Department representative" means an employee of the department, acting as the authorized agent of the commissioner in carrying out the responsibilities and duties specified in Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia.
"Director" means the licensee or a person designated by the licensee who oversees the day-to-day operation of the system, including compliance with all minimum standards for licensed family day-care systems.
"Family day-care system" means any person who approves family day-care homes as members of its system; who refers children to available day-care homes in that system; and who through contractual arrangement may provide central administrative functions, including, but not limited to, training of operators of family day-care homes; technical assistance and consultation to operators of family day-care homes; inspection, supervision, monitoring, and evaluation of family day-care homes; and referral of children to available health and social services. (§ 63.2-100 of the Code of Virginia)
"Family day-care system home" means any private family home, which is an approved member of a family day-care system and receives nine or fewer children for care, protection, and guidance during any part of the 24-hour day except children who are related by blood or marriage to the person who maintains the home. Family day-care homes that are members of a licensed day-care system and are approved by that system to care for five or more children are not subject to direct licensure by the department. (§ 63.2-100 of the Code of Virginia)
"Licensee" means any person, association, partnership, or corporation to whom the license is issued.
"Person" means any natural person or any association, partnership, or corporation. For the purpose of these standards public agencies are not included in this definition individual; corporation; partnership; association; limited liability company; local government; state agency, including any department, institution, authority, instrumentality, board, or other administrative agency of the Commonwealth; or other legal or commercial entity that operates or maintains a child welfare agency.
"Referral" means any activity by the family day-care system that provides assistance in locating or arranging day-care for children in homes that have been accepted or approved as members of the system, or in locating or arranging for health or social services from other sources based upon identified needs.
"Sponsor" means an individual, association, partnership, or corporation having the responsibility for planning and operating a family day-care system subject to licensure. The licensee is the sponsor of a family day-care system. (The sponsor may not, in all cases, be the owner of the physical plant including buildings or real estate, or both, in or on which the family day-care system office is located. In these instances the term "sponsor" as defined here and used in this chapter is considered to be the person, partnership, association, or corporation that owns the enterprise rather than the physical plant or real estate, or both.)
C. The license.
1. A license to operate a family day-care system is issued to a specific person, partnership, association, or corporation for an exact location, which will be indicated on the license.
2. The family day-care system shall be operated and conducted in the name of the sponsor or in such name as shall be designated on the application and as indicated on the license.
3. The license expires automatically and is not transferable when there is a change of sponsorship.
4. The current license shall be posted at all times at a place that is conspicuous to the public in the building housing the system office. If the system has more than one office, copies of the current license shall be posted in a place that is conspicuous to the public in each office.
5. An annual license is one issued to a family day-care system when the activities, services, and facilities meet substantially the minimum standards and requirements for a license that are set forth in this chapter and any additional requirements that may be specified in Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia. The annual license is effective for 12 months unless it is sooner revoked or surrendered.
6. When an annual license expires, a provisional license may be issued for any period not to exceed six months if the applicant is temporarily unable to comply with all of the requirements; however, no facility may operate under any such provisional license and renewals of that license for a longer period than six successive months.
7. At the discretion of the commissioner, a conditional license may be issued to operate a new facility in order to permit the applicant to demonstrate compliance with all requirements. A conditional license and any renewal of that shall be for no longer a period than six successive months.
8. Terms of the license.
a. The terms of any license issued include:
(1) The operating name of the family day-care system;
(2) The name of the individual, the partnership, the association, or the corporation to whom the license is issued;
(3) The physical location;
(4) The number of homes that may be under contract to the system;
(5) The period of time for which the license is effective; and
(6) The total number of children who may be referred by the system and be receiving care at any given time in all homes that are members of the system.
b. The terms of the license may include other limitations that the commissioner may prescribe within the context of this chapter.
c. The provisional license cites the standards with which the licensee is not in compliance.
D. The licensing process.
1. Pre-application Preapplication consultation. Upon request, the department's representative will provide consultation to any person seeking information about obtaining a license for a family day-care system. The purpose of such consultation is:
a. To explain standards;
b. To help the potential applicant to explore the operational demands of a licensed family day-care system;
c. To provide assistance in locating sources of information and technical assistance;
d. To alert the potential applicant of the need to determine whether local ordinances will affect the proposed operation (e.g., zoning, business license, etc.); and
e. To provide an on-site onsite visit to a proposed family day-care system office, upon request.
2. The application.
a. The application for a license to operate a family day-care system shall be obtained from the department.
b. The application, together with all required information, shall be submitted to the department at least two months in advance of the planned opening date.
This is required in order that a determination of compliance with the provisions of Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia and with the Standards for Licensed Family Day-Care Systems as set forth in this chapter may be made.
Among other things, the information submitted shall be sufficient to enable the department's representative to determine, during the subsequent investigation, the specific services to be offered, the adequacy of staff to provide these services, the financial capability of the applicant, the character and reputation of the applicant, including the officers and agents of any association, partnership, or corporation as mandated by § 63.2-1702 of the Code of Virginia.
c. The application shall be signed by the individual responsible for the operation of the family day-care system. The application for a family day-care system to be operated by a board shall be signed by an officer of the board, preferably the chairman.
3. The investigation.
a. Following receipt of the application, the department's representative will make an on-site onsite inspection of the proposed office and an investigation of the proposed services, as well as an investigation of the character and reputation of the applicant, and, upon receipt of the initial application, an investigation of the applicant's financial responsibility.
b. Applicants for licensure and licensees shall at all times afford the commissioner reasonable opportunity to inspect all of their facilities, books, and records, and to interview their agents and employees and any person living or participating in such facilities, or under their custody, control, direction, or supervision. (§ 63.2-1706 of the Code of Virginia) The financial records of an initial applicant shall not be subject to inspection if the applicant submits an operating budget and at least one credit reference.
4. Notice to the applicant of commissioner's action. Upon completion of the investigation of the application for a license, the applicant will be notified in writing of the commissioner's decision.
If the license is issued, an accompanying letter will cite any areas of noncompliance with standards. This letter will also include any limitations on the license and may contain recommendations.
If a license is to be denied, the letter will state the reasons for the intent to deny and will set forth the applicant's right to an administrative hearing.
5. Procedures for renewal of annual, provisional, or conditional license. In order to renew an annual, provisional, or conditional license, the licensee must complete the renewal application and return it, together with any required attachments, to the department. In order to assure timely processing, the renewal application should be completed and returned within 10 days after it is received from the department.
The procedure for investigation and issuance or denial of the license as set forth in subdivisions 3 and 4 of this subsection will be followed.
6. Early compliance (replacement of a provisional or conditional license with an annual license).
a. A provisional or conditional license may be voided and an annual license issued when all of the following conditions exist:
(1) The facility complies with all standards listed on the face of the provisional or conditional license well in advance of the expiration date of the provisional or conditional license, and no additional areas of noncompliance exist;
(2) Compliance has been verified by an on-site onsite observation by the department representative or by written evidence provided by the licensee; and
(3) All other terms of the license remain the same.
b. A request to void a provisional or conditional license and to issue an annual license must be made in writing by the licensee to the regional office of the department from which the family day-care system's license to operate was issued.
c. If the request is approved by the department, the effective date of the new annual license will be the same as the beginning date of the provisional or conditional license.
7. Situation requiring a new application. A new application must be filed when sponsorship of the family day-care system changes.
8. Modification.
a. The conditions of the license may be modified during the effective dates of the license with respect to increasing or decreasing the number of homes that may be placed under contract, the number of children who may be referred by the system and be receiving care at a given time, changing the name of the system when there is no change in sponsorship, changing location of the system office, or other conditions caused by changes in staff, program, or facilities.
b. The licensee shall report to the department any contemplated changes in operation that would affect either the terms of the license or the continuing eligibility for a license. (This does not mean the department has to approve changes in staff or program unless they affect the terms of the license or continuing eligibility.)
c. This information shall be submitted in writing by the licensee to the regional office of the department from which the system's license to operate was issued.
d. The department will then determine whether such changes may be approved and the license modified accordingly or whether a new application must be filed.
9. Determination of continued compliance. In order to determine continued compliance with standards during the effective dates of the license, the department's representative will make announced and unannounced visits to the office or offices of the system and may make such visits to homes that are members of the system.
10. Complaint investigation.
a. The department has the responsibility to investigate any complaints regarding alleged violations of minimum standards for licensed family day-care systems and provisions of Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia.
b. The licensee has the responsibility to investigate any complaints regarding any family day-care home that is approved as a member of its system. (See 22VAC40-120-50 C.) At its discretion the department may also investigate complaints against individual homes.
11. Revocation. Any license may be revoked for failure to maintain these standards or for violation of the provisions of Chapter 17 (§ 63.2-1700 et seq.) of Title 63.2 of the Code of Virginia.
12. Appeals. The applicant or licensee has the right to request an administrative hearing regarding any denial or revocation of a license, in accordance with the provisions of the Administrative Process Act (§ 2.2-4000 et seq. of the Code of Virginia).
Following the receipt of the final order that transmits the department's decision after the administrative hearing, the applicant/licensee applicant or licensee has the right to appeal to a court of record in accordance with § 63.2-1710 of the Code of Virginia.
VA.R. Doc. No. R18-5149; Filed August 18, 2017, 10:11 a.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
REGISTRAR'S NOTICE: The
State Board of Social Services is claiming an exemption from Article 2 of the
Administrative Process Act in accordance with § 2.2-4006 A 4 a of the Code of
Virginia, which excludes regulations that are necessary to conform to changes
in Virginia statutory law or the appropriation act where no agency discretion
is involved. The State Board of Social Services will receive, consider, and
respond to petitions by any interested person at any time with respect to
reconsideration or revision.
Title of Regulation: 22VAC40-131. Standards for
Licensed Child-Placing Agencies (amending 22VAC40-131-10).
Statutory Authority: §§ 63.2-217 and 63.2-1734 of
the Code of Virginia.
Effective Date: October 19, 2017.
Agency Contact: Tammy Trestrail, Department of Social
Services, 801 East Main Street, Richmond, VA 23219, telephone (804) 726-7132,
or email tammy.trestrail@dss.virginia.gov.
Summary:
The amendment conforms the definition of
"licensee" to the definition of "person who operates or
maintains a child welfare agency" under § 63.2-1701 of the
Code of Virginia, pursuant to Chapter 196 of the 2017 Acts of Assembly, to
include an individual; corporation; partnership; association; limited liability
company; local government; state agency, including any department, institution,
authority, instrumentality, board, or other administrative agency of the
Commonwealth; or other legal or commercial entity that operates or maintains a
child welfare agency.
Part I
General Provisions
22VAC40-131-10. Definitions.
"Adoptive home" means any family home selected and
approved by a parent, local board or a licensed child-placing agency for the
placement of a child with the intent of adoption.
"Adoptive parent" means any person selected and
approved by a parent or a child-placing agency for the placement of a child
with the intent of adoption.
"Adoptive placement" means arranging for the care
of a child who is in the custody of a child-placing agency in an approved home
for the purpose of adoption.
"Adult" means any person 18 years of age or older.
"Annual" means within 13 months of the previous
event or occurrence.
"Applicant" means an individual or couple applying
to be approved as a resource, foster, adoptive, treatment foster, or short-term
foster family home provider.
"Background check" means a sworn statement or
affirmation disclosing whether the individual has a criminal conviction, is the
subject of any pending criminal charges within or outside the Commonwealth of
Virginia and is the subject of a founded complaint of abuse or neglect within
or outside the Commonwealth; criminal history record information; child abuse
and neglect central registry search; and any other requirement of 22VAC-40-191
22VAC40-191, Background Checks for Child-Welfare Child Welfare
Agencies, and §§ 63.2-1721 and 63.2-901.1 of the Code of Virginia.
"Behavior support" means those principles and
methods employed by a provider to help a child or youth achieve positive
behavior and to address and correct a child's or youth's inappropriate behavior
in a constructive and safe manner in accordance with goals of the child's or
youth's service or treatment plan and the safety of the child or youth and
others.
"Birth parent" means the biological parent of a
child and, for the purposes of adoptive placement, means parents by previous
adoption.
"Caretaker" means any individual having the
responsibility of providing care for a child and includes the following: (i) a
parent or other person legally responsible for the child's care; (ii) any other
person who has assumed caretaking responsibility by virtue of an agreement with
the legally responsible person; (iii) a person responsible by virtue of their
position of conferred authority; and (iv) adult persons residing in the home
with the child.
"Career and technical education" means organized
sequential educational activities and courses that provide individuals with
academic and relevant technical knowledge and skills needed to prepare for
further education and careers in current or emerging professions.
"Case management" means an activity that assists
individuals eligible for Medicaid in gaining and coordinating access to
necessary care and services appropriate to his needs. Case management
activities are provided in treatment foster care.
"Casework" means provision of direct services or
treatment with an individual or several individuals, and intervention in the
situation on the client's behalf.
"Casework staff" means an individual hired to
perform casework services who has at least a baccalaureate degree with relevant
experience required in this chapter.
"Child" means any natural person under younger
than 18 years of age.
"Child-placing activities" means the activities
involved in the placement of children in foster or adoptive family homes; and
children or youth in children's residential facilities or independent living
arrangements. The following activities and actions are integral components of a
Virginia-licensed child-placing program and when performed in Virginia, these
components are regulated under this chapter:
1. The provision of counseling to biological parents,
including assisting parents to formulate a plan for the care and/or, placement,
or both of their child;
2. The acceptance of a child's custody for placement purposes;
3. Assessing a child's service and placement needs;
4. Performing home studies;
5. Selecting and approving applicants for resource, foster,
treatment foster, or short-term foster care and adoption placements; and
approving independent living placements and services;
6. Matching a child with an approved family or licensed
children's residential facility;
7. Making a placement of a child in a resource, foster,
treatment foster, or short-term foster care home; an independent living
arrangement; or children's residential facility selected for that child;
8. Casework and supervision of children in foster care,
adoption and independent living, including counseling the child, the
biological, adoptive parents, or other persons; and consultation with foster
parents and agencies holding custody of the child; and
9. Providing documentation to finalize adoptions and providing
post-placement adoption and supervision services or making referrals to
appropriate resources for such services.
"Child-placing agency" means any person who places
children in foster homes, adoptive homes, or independent living arrangements
pursuant to § 63.2-1819 of the Code of Virginia; or a local board that
places children in foster or adoptive homes pursuant to §§ 63.2-900,
63.2-903, and 63.2-1221 of the Code of Virginia. Officers, employees, or agents
of the Commonwealth of Virginia or any locality acting within the scope of
their authority as such, who serve as or maintain a child-placing agency, shall
not be required to be licensed.
"Child's family" means the birth or adoptive
parents, legal guardians, or family to whom the child may return.
"Commissioner" means the Commissioner of the
Department of Social Services, his designee, or his authorized representative.
"Complaint" means an accusation that a facility
that is subject to licensure is operating without a license or that a licensed
facility is not in compliance with licensing standards or law.
"Corporal punishment" means punishment administered
through the intentional infliction of pain or discomfort to the body through
(i) actions such as, but not limited to, striking or hitting with any part of
the body or with an implement; (ii) pinching, pulling, or shaking; or (iii) any
similar action that normally inflicts pain or discomfort.
"Department" means the State Department of Social
Services.
"Dual approval process" means a process that
includes a home-study, mutual selection, interviews, training, and background
checks completed on all applicants to be considered for approval as a resource,
foster, or adoptive family home provider.
"Emergency placement" means the placement of a
child where the local department of social services placing the child has
within the past 72 hours removed the child from his home or previous placement
due to abuse or neglect or other emergency.
"Employee," "staff," or "staff
person" means a person working for the licensee who is compensated or has
a financial interest in the business of the licensee, regardless of role,
service, age, function, or duration of employment with the licensee. Employee,
staff, or staff person also includes persons hired through a contract to
provide services for the licensee.
"Foster care placement" means placement of a child
through (i) an agreement between the parents or guardians and the local board
where the legal custody remains with the parents or guardians or (ii) an
entrustment or commitment of the child to the local board or licensed
child-placing agency.
"Foster care services" means the provision of a
full range of casework, treatment, and community services, including but not
limited to independent living services, for a planned period of time to a
child who is abused or neglected as defined in § 63.2-100 of the Code of
Virginia or in need of services as defined in § 16.1-228 of the Code of
Virginia and his family when the child (i) has been identified as needing
services to prevent or eliminate the need for foster care placement, (ii) has
been placed through an agreement between the local board of social services and
the parents or guardians where legal custody remains with the parents or
guardians, or (iii) has been committed or entrusted to a local board of social
services or licensed child-placing agency.
"Foster home" means the place of residence of any
natural person in which any child, other than a child by birth or adoption of
such person, resides as a member of the household.
"Foster parent" means an approved provider who
gives 24-hour substitute family care, room and board, and services for children
committed or entrusted to a child-placing agency.
"Independent living arrangement" means the
placement of a child at least 16 years of age who is in the custody of a local
board or licensed child-placing agency and has been placed by the local board
or licensed child-placing agency in a living arrangement in which he does not
have daily substitute parental supervision.
"Independent living services" means services and
activities provided to a child in foster care 14 years of age or older who was
committed or entrusted to a local board of social services, child welfare
agency, or private child-placing agency. Independent living services may also
include services and activities provided to a person who was in foster care on
his 18th birthday and has not yet reached the age of 21 years. Such services shall
include counseling, education, housing, employment, money management skills
development, access to essential documents, and other appropriate services to
help children or youth and persons prepare for self-sufficiency.
"In-service training" means the on-going
instruction received by providers after they complete their pre-service
training.
"Intercountry placement" means the arrangement for
the care of a child in an adoptive home or foster care placement into or out of
the Commonwealth by a licensed child-placing agency, court, or other entity
authorized to make such placements in accordance with the laws of the foreign
country under which it operates.
"Interstate Compact on the Placement of Children"
means a uniform law enacted by all 50 states, the District of Columbia, and the
U.S. Virgin Islands that establishes orderly procedures for the interstate
placement of children and sets responsibility for those involved in placing
those children.
"Licensee" means the individual, corporation,
partnership, association, limited liability company, trust, business trust,
public entity, or any other legal entity recognized by the Virginia State
Corporation Commission,; local government; state agency, including
any department, institution, authority, instrumentality, board, or other
administrative agency of the Commonwealth; or other legal or commercial entity
to whom the department issues a license and who is legally responsible for
compliance with the regulations and statutory requirements related to the
child-placing agency.
"Licensing representative" means an agent
authorized by the commissioner to carry out the responsibilities and duties
specified in Subtitle IV (§§ 63.2-1700 et seq. and 63.2-1800 et seq.) of
Title 63.2 of the Code of Virginia.
"Local board" means the local board of social
services representing one or more counties or cities.
"Local department" means the local department of
social services of any county or city in this Commonwealth.
"Mental abuse" means that which occurs when a
caretaker creates or inflicts, threatens to create or inflict, or allows to be
created or inflicted upon a child a mental injury by other than accidental
means or creates a substantial risk of impairment of mental functions.
"Mutual selection" means a method within the dual
approval process that encourages collaboration by and between both (i) the
applicant applying for approval as a resource, foster, adoptive, treatment
foster, or short-term foster home provider; and (ii) the child-placing agency who
that is processing the application. It allows both parties the ability
to gather information necessary to make an informed decision about whether the
applicant has a continued interest in and would be ready to accept a child into
his home if it is determined that he meets all criteria to be an approved home
provider. The child-placing agency makes the final determination regarding
approval or disapproval of the applicant.
"Parent" means the birth or adoptive parent of a
child.
"Parental placement" means locating or effecting
the placement of a child or the placing of a child in a family home by the
child's parent or legal guardian for the purpose of foster care or adoption.
"Permanent entrustment agreement" means an
agreement that provides for the termination of all parental rights and
responsibilities with respect to the child to be placed for adoption.
"Permanent foster care placement" means the place
in which a child has been placed pursuant to the provisions of
§§ 63.2-900, 63.2-903, and 63.2-908 of the Code of Virginia with the
expectation and agreement between the placing agency and the place of permanent
foster care that the child shall remain in the placement until he reaches the
age of majority unless modified by court order or unless removed pursuant to
§ 16.1-251 or 63.2-1517 of the Code of Virginia. A permanent foster care
placement may be a place of residence of any natural persons deemed appropriate
to meet a child's needs on a long-term basis.
"Physical abuse" means abuse that occurs when a
caretaker creates or inflicts, threatens to create or inflict, or allows to be
created or inflicted upon a child a physical injury by other than accidental
means; or creates a substantial risk of death, disfigurement, or
impairment of bodily functions.
"Physical neglect" means the failure to provide
food, clothing, shelter, or supervision for a child to the extent that the
child's health or safety is endangered. This also includes abandonment and
situations where the parent or caretaker's own incapacitating behavior or absence
prevents or severely limits the performing of child caring tasks pursuant to
§ 63.2-100 of the Code of Virginia.
"Physical restraint" means use of a physical
intervention or "hands-on" hold to prevent an individual from moving
his body when that individual's behavior places him or others at imminent risk.
"Placing agency" means the child-placing agency
that placed the child with the licensee.
"Pre-service training" means the instruction
received by providers during the initial approval process.
"Provider" means an individual approved as a
resource, foster, adoptive, treatment foster, or short-term foster parent or
family.
"Records" means the written information assembled
in a file relating to the child-placing agency; staff; volunteers; child;
child's family; and resource, foster, adoptive, treatment foster, and
short-term foster family home providers.
"Resource parent" means an approved provider who is
committed to support reunification and who is prepared to adopt the child if
the child and family do not reunify.
"Seclusion" means the involuntary placement of a
child alone in a locked room or secured area from which he is physically
prevented from leaving.
"Serious incident reports" means a written report
detailing the child's accidents or injuries that require medical attention
beyond minor first aid care, criminal activity, and incidents requiring police
intervention.
"Service plan" means a written document that
describes the programs, care, services, and other support that will be offered
to the child and his parents and other prior custodians pursuant to
§ 16.1-281 of the Code of Virginia.
"Sexual abuse" means any act of sexual exploitation
or any sexual act upon a child in violation of the law that is committed or
allowed to be committed by the child's parents or other persons responsible for
the care of the child pursuant to § 63.2-100 of the Code of Virginia.
"Short-term foster care" means a licensee-offered
service that is designed to provide crisis or alternate planned-support relief
for up to 30 consecutive calendar days to resource, foster, adoptive, or
treatment foster family home providers; or biological families through
substitute care placement arrangements for children. The substitute-care
placement environments used shall be limited to provider home environments that
have been approved.
"Special needs" means (i) a physical, mental, or
emotional condition existing prior to adoption; (ii) hereditary tendency,
congenital problem, or birth injury leading to substantial risk of future
disability; or (iii) individual circumstances of the child related to age,
racial, or ethnic background or close relationship with one or more siblings. A
child with special needs is any child for whom it has been determined unlikely
that he will be adopted within a reasonable period of time due to one or more
of the factors described in clause (i), (ii), or (iii) of this definition and
the child is in the custody of a local board or licensed child-placing agency.
A special needs child includes children who have factors described in clause
(i) and (ii) of this definition present at the time of adoption but not
diagnosed until after entry of the final order of adoption and no more than one
year has elapsed.
"State Board" means the State Board of Social
Services.
"Treatment" is the coordinated provision of
services and use of professionally developed and supervised interventions designed
to produce a planned outcome in a person's behavior, attitude, emotional
functioning, or general condition.
"Treatment foster care" is a community-based
program where services are designed to address the special needs of children
and families. Services to children are delivered primarily by treatment foster
parents who are trained, supervised, and supported by child-placing agency
staff. Treatment is primarily foster family based, and is planned and
delivered by a treatment team. Treatment foster care focuses on a continuity of
services, is goal directed and results oriented, and emphasizes permanency
planning for the child in care.
"Treatment foster parent" means a provider,
approved by the licensed or certified child-placing agency, who is trained to provide
treatment foster care services.
"Treatment team" means the group that provides
mutual support; evaluates treatment; and designs, implements, and revises the
treatment and service plan. Treatment team members are persons directly
involved with the child and shall, unless otherwise indicated, consist of the
child; professional child-placing agency staff; other professionals; the
child's family members, where appropriate; and the treatment foster parents.
"Youth" means persons between the ages of 16 and 18
years who are in foster care and persons between the ages of 18 to 21 years
who are former foster care children and are transitioning from foster care to
self-sufficiency.
VA.R. Doc. No. R18-5150; Filed August 18, 2017, 10:12 a.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
REGISTRAR'S NOTICE: The
State Board of Social Services is claiming an exemption from Article 2 of the
Administrative Process Act in accordance with § 2.2-4006 A 4 a of the Code of
Virginia, which excludes regulations that are necessary to conform to changes in
Virginia statutory law or the appropriation act where no agency discretion is
involved. The State Board of Social Services will receive, consider, and
respond to petitions by any interested person at any time with respect to
reconsideration or revision.
Title of Regulation: 22VAC40-151. Standards for
Licensed Children's Residential Facilities (amending 22VAC40-151-10).
Statutory Authority: §§ 63.2-217 and 63.2-1737 of
the Code of Virginia.
Effective Date: October 19, 2017.
Agency Contact: Tammy Trestrail, Department of Social
Services, 801 East Main Street, Richmond, VA 23219, telephone (804) 726-7132,
or email tammy.trestrail@dss.virginia.gov.
Summary:
The amendments conform the definitions of
"applicant" and "'provider' or 'licensee' or 'sponsor'"
with the definition of "person who operates or maintains a child welfare
agency" under § 63.2-1701 of the Code of Virginia, pursuant to Chapter 196
of the 2017 Acts of Assembly, to include an individual; corporation;
partnership; association; limited liability company; local government; state
agency, including any department, institution, authority, instrumentality,
board, or other administrative agency of the Commonwealth; or other legal or
commercial entity that operates or maintains a child welfare agency.
22VAC40-151-10. Definitions.
The following words and terms when used in this chapter shall
have the following meanings unless the context clearly indicates otherwise:
"Allegation" means an accusation that a facility is
operating without a license or receiving public funds for services it is not
certified to provide.
"Allowable variance" means temporary or permanent
waiver of compliance with a standard or portion of a standard, or permission to
meet the intent of the standard by a method other than that specified in the
standard, when the regulatory authority, in its sole discretion, determines (i)
enforcement will create an undue hardship and (ii) resident care will not be
adversely affected.
"Annual" means within 13 months of the previous
event or occurrence.
"Applicable state regulation" means any regulation
that the promulgating state agency determines applies to the facility. The term
includes, but is not necessarily limited to, modules, standards, and
other regulations promulgated by the Departments of Education; Health; Housing
and Community Development; Social Services; or other state agencies.
"Applicant" means the person, any
individual; corporation,; partnership,;
association,; limited liability company or public;
local government; state agency, including any department, institution,
authority, instrumentality, board, or other administrative agency of the
Commonwealth; or other legal or commercial entity that has applied for a
license or certificate.
"Aversive stimuli" means physical forces (e.g.,
sound, electricity, heat, cold, light, water, or noise) or substances (e.g.,
hot pepper, pepper sauce, or pepper spray) measurable in duration and intensity
that when applied to a resident, are noxious or painful to the individual, but
in no case shall the term "aversive stimuli" include striking or
hitting the individual with any part of the body or with an implement or
pinching, pulling, or shaking the resident.
"Behavior support" means those principles and
methods employed by a provider to help a child achieve positive behavior and to
address and correct a child's inappropriate behavior in a constructive and safe
manner in accordance with written policies and procedures governing program
expectations, treatment goals, child and staff safety and security, and the
child's service plan.
"Behavior support assessment" means identification
of a resident's behavior triggers, successful intervention strategies, anger
and anxiety management options for calming, techniques for self-management, and
specific goals that address the targeted behaviors that lead to emergency
safety interventions.
"Body cavity search" means any examination of a
resident's rectal or vaginal cavities, except the performance of medical
procedures by medical personnel.
"Case record" or "record" means
up-to-date written or electronic information relating to one resident. This
information includes social data, agreements, all correspondence relating to
care of the resident, service plans with periodic revisions, aftercare plans
and discharge summary, and any other data related to the resident.
"Child" means any person legally defined as a child
under state law. The term includes residents and other children coming in
contact with the resident or facility (e.g., visitors). When the term is used,
the requirement applies to every child at the facility regardless of whether
the child has been admitted to the facility for care (e.g., staff/child staff
to child ratios apply to all children present even though some may not be
residents).
"Child-placing agency" means any person who places
children in foster homes, adoptive homes or independent living arrangements
pursuant to § 63.2-1819 of the Code of Virginia or a local board that
places children in foster homes or adoptive homes pursuant to §§ 63.2-900,
63.2-903 and 63.2-1221 of the Code of Virginia.
"Children's residential facility" or
"facility" means any facility, child-caring institution, or group
home that is maintained for the purpose of receiving children separated from
their parents or guardians for full-time care, maintenance, protection and
guidance, or for the purpose of providing independent living services to
persons between 18 and 21 years of age who are in the process of transitioning
out of foster care. Children's residential facility shall not include:
1. A licensed or accredited educational institution whose
pupils, in the ordinary course of events, return annually to the homes of their
parents or guardians for not less that than two months of summer
vacation;
2. An establishment required to be licensed as a summer camp
by § 35.1-18 of the Code of Virginia;
3. A licensed or accredited hospital legally maintained as
such; and
4. Any facility licensed by the Department of Social Services
as a child caring institution as of January 1, 1987, and that receives no
public funds.
"Complaint" means an accusation against a licensed
or certified facility regarding an alleged violation of standards or law.
"Contraband" means any item prohibited by law or by
the rules and regulations of the agency, or any item that conflicts with the
program or safety and security of the facility or individual residents.
"Corporal punishment" means punishment administered
through the intentional inflicting of pain or discomfort to the body through
actions such as, but not limited to (i) striking or hitting with any part of
the body or with an implement; (ii) pinching, pulling, or shaking; or (iii) any
similar action that normally inflicts pain or discomfort.
"Corrective action plan" means violations
documented by the department and the facility's submitted pledged corrective
action to the documented violations cited by the regulatory authority.
"Day" means calendar day unless the context clearly
indicates otherwise.
"Department" means the State Department of Social
Services.
"Electronic record" means a record created,
generated, sent, communicated, received, or stored by electronic means.
"Emergency" means a sudden, generally unexpected
occurrence or set of circumstances demanding immediate action. Emergency does
not include regularly scheduled time off for permanent staff or other
situations that should reasonably be anticipated.
"Emergency admission" means the sudden, unplanned,
unexpected admittance of a child who needs immediate care except
self-admittance to a temporary emergency shelter facility or a court-ordered
placement.
"Goal" means expected results or conditions that
usually involve a long period of time and that are written in behavioral terms
in a statement of relatively broad scope. Goals provide guidance in
establishing specific short-term objectives directed toward the attainment of
the goal.
"Good character and reputation" means findings have
been established and knowledgeable and objective people agree that the
individual maintains business or professional, family, and community
relationships that are characterized by honesty, fairness, truthfulness, and
dependability, and has a history or pattern of behavior that demonstrates that
the individual is suitable and able to care for, supervise, and protect
children. Relatives by blood or marriage, and persons who are not knowledgeable
of the individual, such as recent acquaintances, shall not be considered objective
references.
"Group home" means a children's residential
facility that is a community-based, home-like single dwelling, or its
acceptable equivalent, other than the private home of the operator, and serves
up to 12 residents.
"Health record" means the file maintained by a
provider that contains personal health information.
"Human research" means any systematic investigation
including research development, testing, and evaluation, utilizing human
subjects, that is designed to develop or contribute to generalized knowledge.
Human research shall not include research exempt from federal research
regulations pursuant to 45 CFR 46.101(b).
"Immediately" means directly without delay.
"Independent living program" means a
competency-based program that is specifically approved by the department to
provide the opportunity for the residents to develop the skills necessary to
live successfully on their own following completion of the program.
"Independent living services" means those services
and activities designed to assist in self-sufficiency preparation of children
aged 14 and older or individuals who have turned 18 but not yet turned 21 years
old. Such services shall include but not be limited to counseling,
education, housing, employment, and money management, skills development,
and access to essential documents and other appropriate services.
"Individualized service plan" means a written plan
of action developed, and modified at intervals, to meet the needs of a specific
resident. It specifies measurable short-term and long-term goals,
objectives, strategies and time frames timeframes for reaching
the goals, and the individuals responsible for carrying out the plan.
"Legal guardian" means the natural or adoptive
parents or other person, agency, or institution that has legal custody of a
child.
"License" means a document verifying approval to
operate a children's residential facility and that indicates the status of the
facility regarding compliance with applicable state regulations.
"Live-in staff" means staff who are required to be
on duty for a period of 24 consecutive hours or more during each work week.
"Living unit" means the space in which a particular
group of children in care of a residential facility reside. A living unit
contains sleeping areas, bath and toilet facilities, and a living room or its
equivalent for use by the residents of the unit. Depending upon its design, a
building may contain one living unit or several separate living units.
"Mechanical restraint" means the use of an approved
mechanical device that involuntarily restricts the freedom of movement or
voluntary functioning of a limb or portion of a person's body as a means to
control his physical activities when the individual receiving services does not
have the ability to remove the device.
"Medication error" means an error made in
administering a medication to a resident, including the following: (i)
the wrong medication is given to a resident; (ii) the wrong resident is given
the medication; (iii) the wrong dosage is given to a resident; (iv) medication
is given to a resident at the wrong time or not at all; and (v) the proper
method is not used to give the medication to a resident. A medication error
does not include a resident's refusal of offered medication.
"Objective" means expected short-term results or
conditions that must be met in order to attain a goal. Objectives are stated in
measurable, behavioral terms and have a specified time for achievement.
"On duty" means that period of time during which a
staff person is responsible for the supervision of one or more children.
"Parent" means a natural or adoptive parent.
"Parent" means either parent unless the facility has been provided
documentation that there is a legally binding instrument, a state law or a
court order governing such matters as divorce, separation, or custody, that
provides to the contrary.
"Pat down" means a thorough external body search of
a clothed resident.
"Personal health information" means the information
that encompasses the universe of oral, written or otherwise recorded
information that is created or received by an entity relating to either an
individual's physical or mental health or the provision of or payment for
health care to an individual.
"Pharmacological restraint" means the use of a
medication that is administered involuntarily for the emergency control of an
individual's behavior when the individual's behavior places him or others at
imminent risk and the administered medication is not a standard treatment for
the individual's medical or psychiatric condition.
"Physical restraint" (also referred to as a
"manual hold") means use of a physical intervention or
"hands-on" hold to prevent an individual from moving his body when
that individual's behavior places him or others at imminent risk.
"Placement" means an activity by any person that
provides assistance to a parent or legal guardian in locating and effecting the
movement of a child to a foster home, adoptive home, or children's residential
facility.
"Premises" means the tracts of land on which any
part of a residential facility for children is located and any buildings on
such tracts of land.
"Provider" or "licensee" or
"sponsor" means the person individual; corporation;
partnership; association; or public limited liability company; local
government; state agency, including any department, institution,
authority, instrumentality, board, or other administrative agency of the
Commonwealth; or other legal or commercial entity to whom a license is
issued and who is legally responsible for compliance with the regulatory and
statutory requirements relating to the facility.
"Resident" means a person admitted to a children's
residential facility for supervision, care, or training on a 24-hour per day
basis.
"Rest day" means a period of not less than 24
consecutive hours during which a staff person has no responsibility to perform
duties related to the facility.
"Routine admission" means the admittance of a child
following evaluation of an application for admission and execution of a written
placement agreement.
"Rules of conduct" means a listing of a facility's
rules or regulations that is maintained to inform residents and others about
behaviors that are not permitted and the consequences applied when the
behaviors occur.
"Sanitizing agent" means any substance approved by
the Environmental Protection Agency to destroy bacteria.
"Seclusion" means the involuntary placement of an
individual alone, in an area secured by a door that is locked or held
shut by a staff person by physically blocking the door or by any other physical
or verbal means so that the individual cannot leave it.
"Self-admission" means the admittance of a child
who seeks admission to a temporary emergency shelter facility as permitted by
Virginia statutory law without completing the requirements for "routine
admission."
"Severe weather" means extreme environment or
climate conditions that pose a threat to the health, safety, or welfare of
residents.
"Standard" means a statement that describes in
measurable terms a required minimum performance level. The term "standard"
and the term "regulation" may be used interchangeably.
"Strategies" means a series of steps and methods
used to meet goals and objectives.
"Strip search" means a visual inspection of the
body of a resident when that resident's outer clothing or total clothing is
removed and an inspection of the removed clothing. Strip searches are conducted
for the detection of contraband.
"Structured program of care" means a comprehensive
planned daily routine, including appropriate supervision that meets the
needs of each resident both individually and as a group.
"Student/intern" means an individual who
simultaneously is affiliated with an educational institution and a residential
facility. Every student/intern who is not an employee is either a volunteer or
contractual service provider depending upon the relationship among the
student/intern, educational institution, and facility.
"Substantial compliance" means that while there may
be noncompliance with one or more standards that represents minimal risk,
compliance clearly and obviously exists with most of the standards as a whole.
"Target population" means individuals with a similar,
specified characteristic or disability.
"Temporary contract worker" means an individual who
is not a direct salaried employee of the provider but is employed by a third
party and is not a consistently scheduled staff member.
"Temporary emergency shelter facility" means an
emergency shelter specifically approved to provide a range of services, as
needed, on an individual basis not to exceed 90 days, except that this term
does not include secure detention facilities.
"Therapy" means provision of direct diagnostic,
preventive and treatment services where functioning is threatened or affected
by social and psychological stress or health impairment.
"Time out" means the involuntary removal of a
resident by a staff person from a source of reinforcement to a different open
location for a specified period of time or until the problem behavior has
subsided to discontinue or reduce the frequency of problematic behavior.
"Volunteers" means any individual or group who of
their own free will, and without any financial gain, provides goods and
services to the program without compensation.
"Wilderness program" means a facility specifically
approved to provide a primitive camping program with a nonpunitive environment
and an experience curriculum for residents nine years of age and older who
cannot presently function in home, school, or community. In lieu of or in
addition to dormitories, cabins or barracks for housing residents, primitive
campsites are used to integrate learning, mentoring, and group process with
real living needs and problems for which the resident can develop a sense of
social responsibility and self worth.
VA.R. Doc. No. R18-5151; Filed August 18, 2017, 10:10 a.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
REGISTRAR'S NOTICE: The
State Board of Social Services is claiming an exemption from Article 2 of the
Administrative Process Act in accordance with § 2.2-4006 A 3 of the Code of
Virginia, which excludes regulations that consist only of changes in style or
form or corrections of technical errors. The State Board of Social Services
will receive, consider, and respond to petitions by any interested person at
any time with respect to reconsideration or revision.
Title of Regulation: 22VAC40-160. Fee Requirements
for Processing Applications (amending 22VAC40-160-10).
Statutory Authority: §§ 63.2-217 and 63.2-1700 of
the Code of Virginia.
Effective Date: October 19, 2017.
Small Business Impact Review Report of Findings: This
final regulatory action serves as the report of the findings of the regulatory
review pursuant to § 2.2-4007.1 of the Code of Virginia.
Agency Contact: Tammy Trestrail, Program Consultant,
Department of Social Services, 801 East Main Street, Richmond, VA 23219,
telephone (804) 726-7132, or email tammy.trestrail@dss.virginia.gov.
Summary:
The amendments (i) update a Code of Virginia citation due
to the recodification of Title 63.1 to Title 63.2, and (ii) remove the amount
of the returned check fee because the Virginia Department of Social Services
does not set the fee to be charged for returned checks.
22VAC40-160-10. Fees.
By act of the General Assembly and effective February 1,
1984, the Department of Social Services is authorized to charge fees for processing
applications for licenses (§§ 63.1-174.01 and 63.1-196.5 (§ 63.2-1700
of the Code of Virginia).
Such fees are to be used for the development and delivery of
training for operators and staff of facilities or agencies for adults or
children subject to licensure solely by the Department of Social Services.
Each license and renewal of it may be issued for a period of
up to three successive years. The required fee for each licensed facility or
agency will be based upon its licensed capacity and the length of the total
licensure period. However, the fee will be collected annually and licensees
will be billed each year by the Department of Social Services for the
appropriate portion of the fee. (Example: A facility with a capacity of 55
participants is issued a license for a period of 24 months. The fee for that
facility for the two-year period would be $210. The facility will be charged
$105 at the beginning of the licensure period and billed again for $105 at the
beginning of the second year of licensure.) No fee will be charged directly
following the issuance of a conditional license.
Some programs such as, but not limited to, parks and
recreation programs and summer camps, which operate for less than four months
in a 12-month period, will pay a reduced fee as indicated in the fee schedule
below (short-term programs).
Applicants shall use the following schedule of fees to
determine the correct fee to pay for processing all applications.
|
Schedule of Fees
|
|
Capacity
|
1 year
|
2 years
|
3 years
|
|
1–12
|
$14
|
$28
|
$42
|
|
13–25
|
$35
|
$70
|
$105
|
|
26–50
|
$70
|
$140
|
$210
|
|
51–75
|
$105
|
$210
|
$315
|
|
76–200
|
$140
|
$280
|
$420
|
|
201 & up
|
$200
|
$400
|
$600
|
|
Short-term Programs
|
|
1–50
|
$25
|
$50
|
$75
|
|
51 & up
|
$50
|
$110
|
$150
|
|
Flat Fees
|
|
Family Day Care Systems
|
$70
|
$140
|
$210
|
|
Child Placing Agencies
|
$70
|
$140
|
$210
|
The fee shall be paid by personal check, money order, or
certified check, made payable to "Treasurer of Virginia."
A fee that is incorrect in amount or is made payable other
than to the Treasurer of Virginia will be returned to the applicant. Otherwise,
no fee will be returned or refunded for any reason.
Failure to submit the appropriate fee within the time
frame timeframe specified by the Department of Social Services may
result in negative action against a facility's or agency's license.
A $15 fee will be charged for checks which that
must be returned to the applicant because of insufficient funds.
VA.R. Doc. No. R18-5194; Filed August 18, 2017, 10:13 a.m.
TITLE 22. SOCIAL SERVICES
STATE BOARD OF SOCIAL SERVICES
Final Regulation
Final Regulation
REGISTRAR'S NOTICE: The State Board of Social Services is claiming an exemption from Article 2 of the Administrative Process Act in accordance with § 2.2-4006 A 4 a of the Code of Virginia, which excludes regulations that are necessary to conform to changes in Virginia statutory law or the appropriation act where no agency discretion is involved. The State Board of Social Services will receive, consider, and respond to petitions by any interested person at any time with respect to reconsideration or revision.
Title of Regulation: 22VAC40-294. Applications for Public Assistance (adding 22VAC40-294-10, 22VAC40-294-20).
Statutory Authority: §§ 63.2-217, 63.2-501, and 63.2-501.1 of the Code of Virginia.
Effective Date: October 19, 2017.
Agency Contact: Thomas Steinhauser, Director, Division of Benefit Programs, Department of Social Services, 801 East Main Street, Richmond, VA 23219, telephone (804) 726-7362, FAX (804) 726-7662, or email tom.steinhauser@dss.virginia.gov.
Summary:
The action establishes Applications for Public Assistance (22VAC40-294), requiring (i) local departments of social services or the Commissioner of the Virginia Department of Social Services to provide all Medicaid applicants with information regarding advance health care directives, including information about the purpose and benefits of advance directives and how the applicant can make an advance directive and (ii) local departments of social services to obtain alternate contact information for Medicaid applicants, including the applicant's email address and cell phone number, and the applicant's preferred method of contact, including direct mail, email, text message, or phone call, pursuant to Chapters 106 and 472 of the 2017 Acts of Assembly.
CHAPTER 294
APPLICATIONS FOR PUBLIC ASSISTANCE
22VAC40-294-10. Notice of advance directives.
The local department of social services or the Commissioner of the Virginia Department of Social Services shall provide each Medicaid applicant with information on advance directives, including the purpose, benefits, and how to make an advance directive.
22VAC40-294-20. Alternative contact information.
The local department of social services shall collect from each Medicaid applicant alternative contact information, such as the applicant's email address and cell phone number, and the applicant's preferred method of contact, including direct mail, email, text message, or cell phone.
VA.R. Doc. No. R18-5162; Filed August 18, 2017, 10:08 a.m.